Reviews and Information for ON
Search term may appear only in full report available to members. Join now for full access.
Recalls & Warnings
March 13, 2020
FDA Finds Problems at 52% of Supplement Manufacturing Sites in U.S. and 42% Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2019 (October 1, 2018 - September 30, 2019) of 598 dietary supplement manufacturing facilities in the U.S.
Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

Product Review
Ginger Supplements, Chews & Spices Review
Tests Reveal Best and Worst Ginger Supplements & Spices. Poor Quality and Lead Contamination Discovered in Some Products.

Product Review
Vitamin D Supplements Review (Including Calcium, Magnesium, Vitamin K, and Boron)
Find the Best Vitamin D Supplement and Avoid Problems

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Weight Loss Supplements Review (7-Keto DHEA, Forskolin and Stimulant Blend Supplements)
Choose the Best Weight Loss Supplement. Be Careful With Weight Loss Supplements — Few Pass Quality Testing and Safety Review.

Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

Product Review
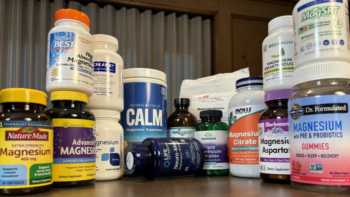
Magnesium Supplements Review (Including Calcium, Vitamins D & K, and Boron)
Find Out What Magnesium Does, Who Needs It, and Our Top Picks Among Supplements
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Recalls & Warnings
March 12, 2016
FDA Finds Problems at 58% of Supplement Manufacturing Sites in U.S. and Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2015 (ending September 30) of 483 dietary supplement manufacturing facilities, showing that most -- 58.2% -- received letters indicating noncompliance with current Good Manufacturing Practices (cGMPs).
Product Review
Apple Cider Vinegar Review — Bottled Liquids and Supplements
Find the Best Apple Cider Vinegar. See Which Apple Cider Vinegar Liquids and Supplements Passed Our Tests of Quality.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
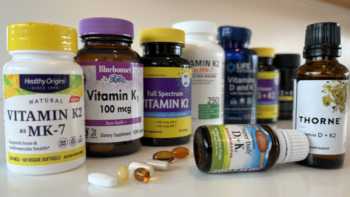
Vitamin K Supplements Review (Including Calcium, Vitamin D, Magnesium & Boron)
Find Out If It's Worth Taking Vitamin K and Which Products Are Best
Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
18% of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and Our Top Picks.

Product Review
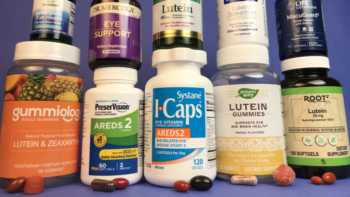
Vision Supplements Review (with Lutein, Zeaxanthin & AREDS2 Formulas)
Find the Best Vision Supplement Based Our Tests
Product Review
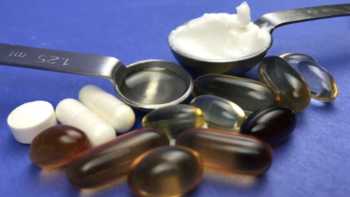
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
Product Review
Red Yeast Rice Supplements Review
50% of Red Yeast Rice Supplements "NOT APPROVED" in CL Tests

Product Review
Glutathione Supplements Review
Learn How Glutathione Products Differ, Which Failed, and Our Top Picks

Product Review
Green Tea Review: Tea Bags, Loose Leaf Tea, Matcha Powders, and Supplements
Some green teas provide barely any green tea polyphenols, while some others are high strength. See the Test Results and Our Top Picks for Green Tea.

Product Review
Probiotic Supplements Review (Including Pet Probiotics)
Probiotics: See What They Really Contain and Our Top Picks

Product Review
SAMe (S-adenosyl-methionine) Supplements Review
Choose the Best SAMe Supplement and Save Money
Product Review
Ginseng Supplements Review
Find the Best Ginseng Supplement. Key "Ginsenosides" Found to Range 10-Fold Across Products.

Product Review
L-Lysine Supplements Review
Read L-Lysine Labels Carefully. Some Can Fool You.
Product Review
Choline and Lecithin Supplements Review (Including Phosphatidylcholine, CDP-Choline, and Alpha-GPC)
Choose the Best Choline Supplement. Find Out How Much Choline Popular Supplements Really Provide.

Product Review
Cinnamon Supplements and Spices Review
CAUTION: Some Cinnamon Products High in Toxin. See Which Passed or Failed and our Top Picks!

Product Review
Menopause Supplements Review (Soy and Red Clover Isoflavones, Black Cohosh) and Progesterone Creams
Choose the Best Menopause Supplement. Find Out Now Which Soy Isoflavone, Red Clover, Black Cohosh, and Progesterone Products Have the Active Compounds You Want!

Product Review
Iron Supplements Review (Iron Pills, Liquids and Chews)
See Which Iron Supplements Are CL's Top Picks for Different Needs

Product Review
Astaxanthin Supplements Review
See Which Astaxanthin Products Passed or Failed CL's Tests

Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

Product Review
Tuna, Salmon, Sardines & Herring Review (Canned and Packaged)
Find Products With the Least Mercury and Most Omega-3s

Product Review
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
Product Review
Potassium Supplements Review
Be Careful with Potassium Supplements! Problems Found. Tests and Reviews of Potassium Supplements & CL's Top Picks.
Product Review
Resveratrol Supplements Review (From Red Wine, Knotweed, and Other Sources)
See Which Resveratrol Supplements Were Best In Our Tests and Comparisons. Learn What Resveratrol Can and Can't Do.

Product Review
Cholesterol-Lowering Supplements Review (Sterols/Stanols and Policosanol)
Find the Best Cholesterol-Lowering Supplement. See Which Plant Sterol and Policosanol Supplements Passed Our Tests and Are Our Top Picks.
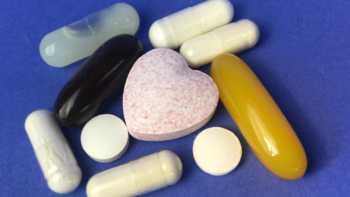
Product Review
Psyllium Fiber Supplements Review
Find the Best Psyllium Fiber Supplement and Avoid Lead.

Product Review
Ashwagandha Supplements Review
Find the Best Ashwagandha Supplement. Only 38% of Ashwagandha Products Pass Tests.

Product Review
Gluten-Free Grains Review (Buckwheat, Quinoa, Sorghum, Millet, and Teff)
31% of Gluten-Free Grains Fail Our Tests Due to Contaminants. Quinoa, Buckwheat, Sorghum, Millet and Teff Tested.

Product Review
Phosphatidylserine (PS) Supplements
Find out if phosphatidylserine helps with memory and which products are best.

Product Review
Alginate Supplements Review (For Reflux)
See Our Top Pick Alginate Supplements For Reducing Symptoms of Gastroesophageal Reflux (GERD)
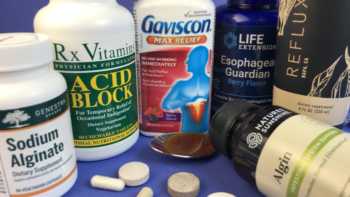
Product Review
Ginkgo (Ginkgo Biloba) Supplements Review
Choose the Best Ginkgo Biloba Supplement. Finding Real Ginkgo Isn't Easy — 60% Fail ConsumerLab's Tests of Quality.

Product Review
Calcium and Bone Health Supplements Review (Including Vitamins D & K, Magnesium and Boron)
See Which Bone Health Supplements Are Top Picks and Which Fail
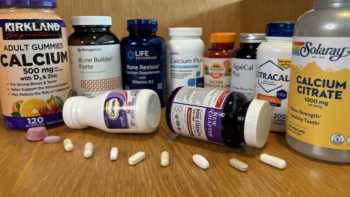
Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

Product Review
L-Theanine Supplements Review
Touted for Stress Relief, Do L-Theanine Supplements Work?
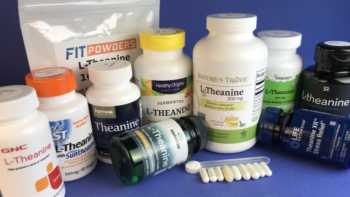
Product Review
Selenium Supplements Review
Choose the Best Selenium Supplement. Find Out If You Need Selenium and Which Supplement Is Our Top Pick.
Product Review
Elderberry Supplements Review
Find the Best Elderberry Supplement. Tests and Reviews of Popular Elderberry Supplements & CL's Top Picks.

Product Review
Acai Berry Supplements and Beverages Review
Find the Best Acai Berry Supplements and Beverages. See Which Acai Berry Supplements and Beverages Passed Our Tests of Quality.
Product Review
Lactose Intolerance Products Review (Lactase Enzyme Supplements and Lactose-Free Milks)
Choose the Best Lactase Enzyme Supplement and Lactose-Free Milk. Find a CL Approved Lactose Intolerance Product.

Product Review
Garlic Supplements Review
Find the Best Garlic Supplements. CL Tests Reveal Big Differences in Garlic Strength -- Some Have Little to No Garlic!.

Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

Product Review
Alpha-Lipoic Acid Supplements Review
Choose the Best Alpha-Lipoic Acid Supplement — See the Amounts of Active "R-Isomer" We Found

Product Review
Prostate Supplements Review (Saw Palmetto and Beta-Sitosterol)
Find the Best Prostate Supplement for Symptoms of BPH. See Which Prostate Supplement Passed or Failed CL's Tests of Quality.
Product Review
Joint Health Supplements for Pets Review (Glucosamine, Chondroitin, MSM & Boswellia)
See Which Pet Supplements for Joint Health Passed Our Tests and Which Didn't!

Product Review
Bilberry Supplements Review
Choose the Best Bilberry Supplement. Some Bilberry Is Not Authentic!

Product Review
Nattokinase Supplements Review
Choose the Best Nattokinase Supplement. CL Tests Reveal Which Nattokinase Suppplements Provide the Best Quality & Value.
Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Product Review
Electrolytes & Sports Drinks Review
See Which Sports Drinks, Powders, and Pills Deliver the Right Electrolytes
Product Review
Garcinia Cambogia (HCA) Supplements Review
Find the Best Garcinia Cambogia Supplement. Choose Carefully! Most Garcinia (HCA) Weight Loss Supplements Lack Listed Amount of Ingredient .

Product Review
Nutrition Bars & Cookies Review (For Energy, Fiber, Protein, Meal Replacement, and Whole Foods)
Find the Best Nutrition Bar or Cookie. ConsumerLab Tests Reveals Not All Nutrition Bars and Cookies Contain What They Claim.

Product Review
Collagen Supplements Review
See our Top Picks for Wrinkles and Joints

Product Review
Echinacea Supplements Review
Many Echinacea Supplements Fail CL's Tests. Make Sure You Know What You're Getting!

Product Review
Green Coffee Bean Extract Supplements Review (for Weight Loss)
Choose the Best Green Coffee Bean Extract. 50% of Green Coffee Bean Extract Supplements Don't Deliver Expected Ingredients.

Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

Product Review
Cranberry Juices and Supplements Review
CL's Tests Show Which Cranberry Juices and Supplements Are Best and Cost the Least

Product Review
CBD Oils, Softgels, Gummies, Creams & Salves Review
See How Much CBD and THC We Found in Products.

Product Review
Lion's Mane and Chaga Supplements Review
Read Labels Carefully -- Many Can Mislead

Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

Product Review
Rolled Oats and Steel-Cut Oats Review
Find the Best Rolled and Steel-Cut Oats -- Beware of Unexpected Gluten.

Product Review
Boron Supplements Review (Including Calcium, Magnesium, and Vitamins D & K)
Is Boron Needed for Bone Health? Find Out and See Our Top Pick Among Supplements
Product Review
Shilajit Supplements Review (Resins and Extracts)
We Discovered Major Differences Among Shilajit Supplements

Product Review
Kelp Supplements Review
Choose the Best Kelp Supplement. Be Cautious With Kelp! Only 50% of Supplements Pass CL's Review.

Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
Product Review
Coconut Waters Review
Best Coconut Water? See Our Tests and Comparisons of Popular Brands Looking at Sugar, Electrolytes, Taste and Price.

Product Review
Milk Thistle Supplements Review
Find the Best Milk Thistle Supplement. Tests and Reviews of Popular Milk Thistle Supplements & CL's Top Picks.

Product Review
Vitamin C Supplements Review
Find the Best Vitamin C Supplements

Product Review
Shelled Walnuts (Halves & Pieces)
See Our Top Picks for Walnuts

Product Review
GABA Supplements Review
Does GABA Work As a Supplement?

Product Review
Plant-Based Milks Review (Almond, Cashew, Coconut, Flax, Hemp, Macadamia, Oat, Pea, and Soy)
Find the Best Non-Dairy Milk Alternative. ConsumerLab Tests Reveal What's Really In Plant-Based Milks.

Product Review
Seaweed Snacks and Foods Review
Tests Reveal Seaweed Snack Risks

Product Review
NAD Booster Supplements Review (NAD+/NADH, Nicotinamide Riboside, and NMN)
How Important Is Boosting NAD+ Levels? Find Out and Learn How Booster Supplements Compare.
Product Review
PQQ (Pyrroloquinoline Quinone) Supplements Review
Learn What PQQ Does and Which PQQ Supplements are Best
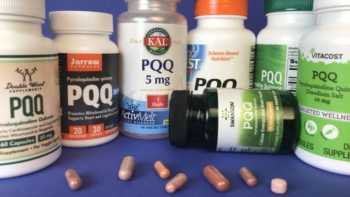
Product Review
DHEA Supplements Review
Choose the Best DHEA Supplement. Beware of Big Differences in Dose and Price.

Product Review
Black Currant, Borage, Evening Primrose, Flax and Hemp Oil Review
Choose the Best Flaxseed and Other Oils Based on Our Tests.

Product Review
D-Mannose Supplements
Find out if D-mannose helps prevent urinary tract infections and how products compare.
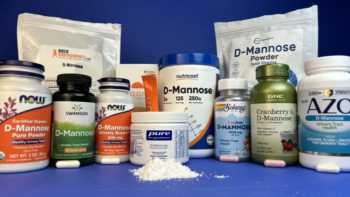
Product Review
Rhodiola Rosea Supplements Review
Do Rhodiola Supplements Help With Depression and Anxiety? Find Out and See Which Rhodiola Supplements Provide the Best Quality & Value.

Product Review
Maca Supplements Review
Choose the Best Maca Supplement. Make Sure Your Maca Supplement Isn't Contaminated With Lead.

Product Review
Sexual Enhancer Supplements Review (with Yohimbe, Horny Goat Weed, Arginine)
Choose the Best Sexual Enhancer Supplement. Only 30% of Selected Sexual Enhancement Supplements Pass Quality Tests.

Product Review
Bone Broth Review
Find the Best Bone Broth. Find Out How Much Collagen and Sodium Is Really In Popular Bone Broths.

Product Review
Vitamin A Supplements Review, Including Beta-Carotene and Cod Liver Oil
Find the Best Vitamin A Supplement. Best Quality Vitamin A Supplements Identified, Including Cod Liver Oil.

Product Review
Prebiotic Supplements Review
You Can't Tell How Much Fiber Is Really In Most Prebiotics Without Testing

Product Review
Taurine Supplements Review for People, Dogs, and Cats
Find the Best Taurine Supplements. Learn When to Use Taurine and Which Supplement is Best.
Product Review
Lycopene Supplements Review
Find the Best Lycopene Supplements. CL Tests Reveal That Not All Lycopene Supplements Contain What They Claim.

Product Review
Chromium Supplements Review
Chromium Supplements Review. Best Chromium Supplements Identified -- By Strength.

Product Review
Chia Seed Review — Whole, Ground, and Sprouted Seed, Seed Flour, & Supplements
Find the Best Chia Seeds, Sprouted Chia Powder, Flour, and Chia Supplements. Tests and Reviews of Chia Seeds & CL's Top Picks.

Product Review
Avocado Oil Review
Find the Best Avocado Oil for Purity, Freshness and Taste. Some Others May Include Rotten Avocado or Other Oils.

Product Review
Saffron Supplements Review
See How Saffron Supplements Compare and What They Do

Product Review
Farro and Bulgur Review
See How Bob's Red Mill Compares to Trader Joe's

Product Review
Olive Leaf Extract Supplements Review
Learn What Olive Leaf Does and the Best Brands

Product Review
Aloe Juices, Gels, and Supplements Review
How Much Aloe is Really in Aloe Products? Find Out and See Our Top Picks.

Product Review
CLA (Conjugated Linoleic Acid) Supplements Review (for Slimming)
Choose the Best CLA Supplement. Not All CLA Supplements Contain What You Expect.

Product Review
Lavender and Tea Tree Essential Oils Review
Oils Tested for Authenticity and Purity

Product Review
Acetyl-L-Carnitine Supplements Review
Choose the Best Acetyl-L-Carnitine Supplement. Don't Overpay! CL Identifies Quality Acetyl-L-Carnitine Supplements at the Best Value.
.jpg?size=small)
Product Review
Low-Dose Lithium Supplements Review
Choose the Best Low-Dose Lithium Supplement. CL Tests Reveal Which Low-Dose Lithium Supplements Offer the Best Quality and Value.

CL Answer
Immunocal is much more expensive than other whey protein isolates - is it worth the extra cost?
Find out how Immunocal, from Immunotec, compares to other whey protein isolates and understand it's costs.
Product Review
Wellbutrin vs. Generic Bupropion Review
Generic Bupropion Is Not Always The Same As Name-Brand Wellbutrin. CL Tests Reveal Differences Between Generic Bupropion and Name-Brand Wellbutrin.
Product Review
St. John's Wort Supplements Review
Find the Best St. John's Wort Supplement. Only 40% of St. John's Wort Supplements Pass Tests & Strength Varies Widely.

Product Review
Pumpkin Seeds (Pepitas) Review
We Identified the Best Pumpkin Seeds

News Release
February 25, 2025
Top-rated Vitamin and Supplement Brands and Merchants for 2025 Based on Consumer Satisfaction
White Plains, New York, February 25, 2025 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 8,400 responses collected in November/December 2024.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
Product Review
Black Seed Oil Review
Find Out Which Black Seed Oil Supplements Passed or Failed Our Tests. See Our Top Picks.

Recalls & Warnings
January 04, 2024
Toxic Herb Found in More Tejocote Root Supplements
On January 3, 2024, the FDA warned consumers not to purchase or use certain tejocote root supplements after FDA laboratory analysis confirmed the products contain yellow oleander (Thevetia peruviana), a toxic herb.
News Release
July 26, 2023
Best Collagen Supplements for Skin and Joints, According to ConsumerLab Tests
White Plains, New York, July 26, 2023 — Collagen supplementation may modestly reduce wrinkles and the appearance of cellulite, and moderately decrease joint and tendon pain and improve flexibility in people with osteoarthritis.
Recalls & Warnings
September 01, 2022
Elite One Source Nutritional Services Warned for Manufacturing, Labeling Violations
On August 5, 2022, the FDA issued a warning letter to dietary supplement manufacturer Nutritional Laboratories International, Inc. (DBA Elite One Source Nutritional Services, Inc.
Product Review
Toprol XL vs. Generic Metoprolol Succinate Extended-release (ER) Tablets Review Article
Choose the Best Blood Pressure Medication. Find Out Why Some Generic Blood Pressure Medications Are Not the Same as the Original & May Increase Blood Pressure or Have Other Disturbing Side Effects.
Product Review
Huperzine A Supplements Review
Choose the Best Huperzine A Supplement. CL Tests Reveal the Best Huperzine A Supplements for Memory.

CL Answer
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
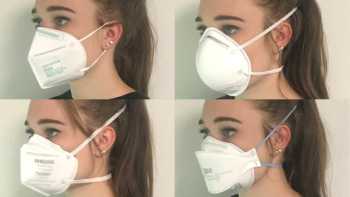
Recalls & Warnings
January 23, 2025
Glutathione Distributor Warned by FDA
On November 13, 2024, the FDA issued a Warning Letter to Western Innovations, Inc.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
July 26, 2023
Gadget Island, Inc. Male Enhancement Supplements Found to Contain Prescription Drugs
On July 21, 2023, the FDA issued a warning letter to Gadget Island, Inc.
Recalls & Warnings
May 04, 2023
FDA Warns MedoLife for Promoting Homeopathic Products to Treat COVID-19, Cancer
On April 19, 2023, the FDA issued a warning letter to Medolife Rx D/B/A MedoLife Corp., AELIA Inc. and Queanta Inc.
Recalls & Warnings
September 10, 2021
FDA Warns Ten Sellers of "Diabetes" Supplements
On September 7, 2021, the FDA issued warning letters to 10 supplement companies that made drug claims by promoting products to treat diabetes and/or lower blood sugar. Five of the products were sold on Amazon as well as on company websites. The products were promoted with statements such as
CL Answer
Does AZO Bladder Control really work for overactive bladder?
AZO Bladder Control information, including results from clinical studies on bladder control, dosage, and safety.

CL Answer
Where to Safely Buy Real Vitamins and Supplements Online, Not Fakes or Counterfeits
ConsumerLab explains how to avoid counterfeit vitamins and supplements when shopping online on Amazon, Walmart.com, and other sites. Learn to identify authorized sites and sellers and avoid fake supplements. Use the brand-by-brand guide to protect yourself from risk.

Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
September 14, 2023
FDA Warns CVS, Walgreens, Similasan & Others for Eye Drop Violations
On September 11, 2023, the FDA issued Warning Letters to the following eight sellers of homeopathic and other types of eye drops regarding the products noted in italics due to a variety of violations of FDA regulations, most notably that they were marked with claims suggesting that they could cure, ...
CL Answer
Does Alpha Brain really improve memory, focus and cognition?
Find out if Alpha Brain really improves memory, focus and cognition. See the clinical evidence and get details about Alpha Brain ingredients, as well as potential side effects and drug interactions. ConsumerLab.com's answer explains.

Recalls & Warnings
March 13, 2025
FTC Sends Refund Checks to Consumers of Pure Green Coffee Supplement
On March 6, 2025, the Federal Trade Commission (FTC) announced it is mailing 39,977 checks totaling more than $905,000 to consumers who purchased Pure Green Coffee, to settle charges the product was promoted with deceptive health claims and marketing practices.
Recalls & Warnings
July 25, 2024
West Coast Laboratories Warned for Manufacturing, Labeling Violations
On March 12, 2024, the FDA issued a Warning Letter to West Coast Laboratories, Inc.
News Release
June 08, 2023
Best Muscle and Workout Supplements With Creatine or BCAAs, According to ConsumerLab Tests
White Plains, New York, June 8, 2023 — Creatine supplements are promoted to increase muscle size, strength, and endurance, while supplements with branched-chain amino acids (BCAAs) are promoted to improve exercise recovery.
Recalls & Warnings
April 17, 2021
NS NY Distributor Inc Male Enhancement Products Recalled Due to Undeclared Drugs
On April 8, 2021, NS NY Distributor Inc issued a recall of all lots of Premium Orgazen 7000 and Ginseng Power 5000 male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
CL Answer
Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Find out if natural remedies & supplements for coronavirus such as zinc, vitamin C, garlic, or elderberry help to prevent or treat COVID-19.
Recalls & Warnings
July 17, 2023
Seller of Ashwagandha, Lion’s Mane & Other Supplements Warned for Manufacturing Violations
On March 17, 2023, the FDA issued a warning letter to Brand Packaging Group, Inc. after FDA inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
July 19, 2023
FDA Warns Seller of Sleepy Time Products and Honey Herbal Syrups for Manufacturing Violations
On June 28, 2023, the FDA issued a warning letter to Eden’s Answers, Inc. after an inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
June 19, 2023
A&Z Pharmaceutical Warned for Promoting Calcium & Vitamin D for Hair Loss, Osteoporosis & Cancer
On June 1, 2023, the FDA issued a warning letter to A&Z Pharmaceutical, Inc. following inspection of the company’s website and social media, which found statements about the company’s Chewable Calcium 600MG with Vitamin D for Kids in Orange Flavor products to be drug claims.
Recalls & Warnings
November 09, 2023
InnoMark Warned for Manufacturing & Misbranding Violations
On September 1, 2023, the FDA issued a Warning Letter to InnoMark, Inc.
Recalls & Warnings
September 06, 2023
WEFUN Capsules Recalled Due to Undeclared Sildenafil
On August 25, 2023, WEFUN Inc. issued a recall of 300 boxes of WEFUN Capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
January 03, 2024
FDA Warns Amazon for Selling Men’s Supplements Containing Prescription Drugs
On December 20, 2023, the FDA issued a Warning Letter to Amazon.com, Inc.
CL Answer
Do detox foot pads or ionic foot baths remove heavy metals or other toxins from the body?
Detox foot pads and ionic foot baths are promoted for removing heavy metals and other toxins from the body. Find out if there is evidence to support these claims.
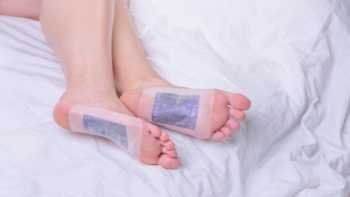
CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

CL Answer
What are the health benefits of coconut oil and medium chain triglycerides, such as those used in Bulletproof Coffee?
Learn the health benefits of coconut oil and medium chain triglycerides (MCTs), including evidence from Bulletproof Coffee, clinical studies on obesity, skin, and Alzheimer's disease.

Recalls & Warnings
October 06, 2005
Deceptive Marketing of “Supreme Greens" -- Settlement with FTC
On October 6, 2005, the Federal Trade Commission (FTC) announced that three individuals and two companies have settled FTC charges over their roles in the deceptive marketing of Supreme Greens, an herbal supplement.
Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
Recalls & Warnings
January 06, 2025
ZOE Prebiotic Blend Recalled Due to Metal Pieces, Stones
On December 4, 2024, Zoe US, Inc. recalled 142 units of ZOE Daily 30+ Prebiotic blend because it may contain foreign objects such as small metal pieces and/or stones.
News Release
February 25, 2023
Top-rated Vitamin and Supplement Brands and Merchants for 2023 Based on Consumer Satisfaction
White Plains, New York, February 25, 2023 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,600 responses collected in November/December 2022.
News Release
June 30, 2022
Best Protein Shakes & Drinks According to ConsumerLab Tests. Lead, Excess Sodium Found in Some
White Plains, New York, June 30, 2022 — Consumers may get more sodium, fat, calories, or even lead, than they bargained for from some protein supplements, according to recent ConsumerLab tests of over 20 popular protein powders and shakes.
News Release
February 25, 2022
Top-rated Vitamin and Supplement Brands and Merchants for 2022 Based on Consumer Satisfaction
White Plains, New York, February 25, 2022 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,049 responses collected in November/December 2021.
News Release
February 25, 2021
Top-rated Vitamin and Supplement Brands and Merchants for 2021 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2021 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,647 responses collected in November 2020.
News Release
February 25, 2020
Top-rated Vitamin and Supplement Brands and Merchants for 2020 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2020 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,782 responses collected in late November and early December 2019.
News Release
October 04, 2019
ConsumerLab Tests Reveal Best Collagen Supplements for Wrinkles and Joints
White Plains, New York, October 4, 2019 — Collagen supplements are promoted to reduce wrinkles and decrease joint pain and stiffness, but do they really work, and if so, which products on the market provide the best quality collagen and the best value? To find out, ConsumerLab carefully ...
News Release
February 25, 2019
Top-rated Vitamin and Supplement Brands and Merchants for 2019 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2019 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,931 responses collected in late November and early December 2018.
News Release
January 03, 2019
ConsumerLab Tests Reveal the Best Protein Powders, Shakes and Drinks -- 20% of Protein Powders and Drinks Fail CL's Tests of Quality
White Plains, New York, January 3, 2019 — Combined with resistance exercise, protein supplements can help athletes build muscle and older people prevent muscle loss. They can also help people with diabetes maintain blood sugar levels when consumed as part of a low-calorie diet.
News Release
February 25, 2018
Top-rated Vitamin and Supplement Brands and Merchants for 2018 Based on Consumer Satisfaction
White Plains, New York, February 25, 2018 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,446 responses collected in late November and early December 2017.
Recalls & Warnings
September 11, 2015
Thirteen Manufacturers Ordered to Stop Selling Devil's Claw Supplements
On September 9, 2015, the New York State Attorney General ordered 13 manufacturers, including Thorne Research Inc., NBTY, Inc. (Puritan's Pride) Inc., and Now Foods, to stop selling devil's claw supplements which were found to contain the incorrect species of the plant.
Recalls & Warnings
April 28, 2020
Ten Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On April 24, 2020, the FTC announced that it sent warning letters to ten multi-level marketing companies for selling products such as essential oils and immune system boosters with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrespresenting potential earnings people ...
Recalls & Warnings
August 17, 2022
Seller of Moringa Tea and Other Herbal Products Warned for Drug Claims, Manufacturing Violations
On July 29, 2022, the FDA issued a warning letter to Deggeh Foods, Inc.
Recalls & Warnings
July 25, 2022
Nature’s Sunshine Protein Powder Recalled
On July 22, 2022, Nature’s Sunshine Products Inc. issued a recall of two AIVIA Whey Protein Powder Herbs meal replacement products due to undeclared milk.
Recalls & Warnings
June 13, 2022
Homeopathic Nasal Spray SnoreStop Recalled Due to Microbial Contamination
On June 9, 2022, Green Pharmaceuticals Inc issued a voluntary nationwide recall of one lot of its SnoreStop NasoSpray, a homeopathic nasal spray promoted to reduce snoring, due to the presence of Providencia rettgeri, a bacterium that can cause severe illness in some people.
Recalls & Warnings
July 14, 2022
FDA Warns Seller of Unauthorized COVID-19 Tests
On June 30, 2022, the FDA issued a warning letter to W.H.P.M, Inc. for distributing COVID antigen tests without approval, clearance, or authorization from the FDA while claiming to mitigate, prevent, treat, diagnose, or cure COVID-19 in people.
Recalls & Warnings
January 19, 2023
Male Sexual Enhancement Supplement Adam’s Secret Found to Contain Prescription Medication
On January 10, 2023, the FDA issued a warning letter to HIS Enterprise Inc dba Adam’s Secret USA, LLC after laboratory analysis found Adam’s Secret Extra Strength 3000 Platinum, Adam’s Secret Extra Strength Blue, Adam’s Secret Extra Strength Purple, Adam's Secret ...
Recalls & Warnings
January 26, 2023
FDA Warns Seller of Canary Seed Omega-3
On November 16, 2022, the FDA issued a warning letter to Evimeria El Aztecano, Inc. following review of the company’s Yerbas Finas Leche de Alpiste con Guanabana y Omega-3 product labeling, which found the product contained less magnesium and potassium than claimed on the label.
Recalls & Warnings
March 30, 2023
Seller of Tollovid Supplements Warned for COVID-19 Claims
On November 7, 2022, the FDA issued a warning letter to Todos Medical Ltd, aka Todos Medical USA Inc, following a review of the company’s websites, social media, and Amazon storefronts, which found statements about the company’s Tollovid 3CL Protease Inhibitor Delayed Release ...
Recalls & Warnings
April 25, 2022
Some OTC Skin Lighteners Contain Potentially Harmful Ingredient, Warns FDA
On April 19th, 2022, the FDA issued warnings to 12 companies for selling over-the-counter (OTC) skin-lightening products containing hydroquinone.
Recalls & Warnings
May 09, 2022
Natural Organics Keto Capsules Recalled Due to Gluten
On March 6, 2022, Natural Organics, Inc. issued a recall of four lots of NaturePlus Keto Living Sugar Control Capsules that were found to contain gluten.
Recalls & Warnings
August 16, 2023
FDA Warns Sun Ten Laboratories for Manufacturing Violations, Drug Claims
On April 7, 2023, the FDA issued a warning letter to STPCA Inc. dba Sun Ten Laboratories because products were found to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
April 27, 2023
Seller of Magtein, Veggie Caps, & More Warned for Drug Claims, Manufacturing Violations
On March 8, 2023, the FDA issued a warning letter to Spartan Enterprises Inc., dba Watershed Wellness Center, after a review of the company’s website found statements about its Dr. Bob’s Naturals Spirulina, Dr. Bob’s Naturals Magtein, Dr.
Recalls & Warnings
April 23, 2021
FDA Warns 5 Sellers of Unapproved COVID-19 Tests
Between March 18 and April 6, 2021, the FDA issued warning letters to five companies for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
News Release
February 25, 2017
Top-rated Vitamin and Supplement Brands and Merchants for 2017 Based on Consumer Satisfaction
White Plains, New York, February 25, 2017 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,505 responses collected in late November and early December 2016.
News Release
December 06, 2016
Which Brands of Alpha Lipoic Acid are Best? -- ConsumerLab.com Tests Lipoic Acid Supplements for Quality, Identifying Top Picks
White Plains, New York, December 6, 2016 — Alpha-lipoic acid supplements may improve insulin sensitivity and blood sugar control in people with type 2 diabetes, reduce symptoms of diabetic peripheral neuropathy, and enhance weight loss when dieting.
News Release
October 11, 2016
What Are the Benefits of Resveratrol and Which Brands Are Best? -- ConsumerLab.com Reviews the Evidence and Tests the Quality of Popular Resveratrol Supplements
White Plains, New York, October 11, 2016 — Resveratrol supplements have been popular since 2006, when studies in animals showed "life-extending" and "endurance-enhancing" effects, among other potential benefits.
News Release
February 25, 2016
Top-rated Vitamin and Supplement Brands and Merchants for 2016 Based on Consumer Satisfaction
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,534 responses collected in November, 2015. Respondents gave ratings for 963 brands and 399 merchants.
News Release
February 25, 2015
Top-rated Vitamin and Supplement Brands and Merchants for 2015 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,329 responses collected in November, 2014. Respondents gave ratings for 1,709 brands and 891 merchants.
News Release
May 07, 2014
Flaxseed and Other Popular Seed Oil Supplements Tested by ConsumerLab.com, But Not All Brands Pass -- Flaxseed, Black Currant, Borage and Evening Primrose Oils Reviewed
White Plains, New York, May 7, 2014 — Plant seed oils are popular supplements that provide omega-3 and omega-6 fatty acids, but which products offer the best value and quality? Recent analyses by ConsumerLab.
News Release
February 12, 2014
Top-rated Vitamin and Supplement Brands and Merchants for 2014 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,326 responses collected in November, 2013. Respondents gave ratings for 1,639 brands and 788 merchants.
News Release
June 11, 2013
31% of Protein Powders and Drinks Fail Tests by ConsumerLab.com
White Plains, New York — June 11, 2013 — What's really in a scoop of "protein powder"? sIn some products, a lot less protein or a lot more carbohydrates than you would expect, and perhaps even a serving of something you don't want -- lead. That's according to recent tests by ConsumerLab.
News Release
February 01, 2013
Top-rated Vitamin and Supplement Brands and Merchants for 2013 Based on Consumer Satisfaction
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,862 responses collected in November, 2012. Respondents gave ratings for 1,438 brands and 851 merchants.
Recalls & Warnings
March 14, 2011
22 Brands of Whey Protein Recalled Due to Salmonella Concern
The FDA posted a recall notice involving 22 brands of whey protein powder due to potential contamination with salmonella. The recall was voluntarily initiated on March 10, 2011 by the manufacturer of the products, Vitalabs, Inc.
CL Answer
Do vitamins, minerals or other supplements lose effectiveness with exposure to high temperatures?
Are vitamins and supplements susceptible to heat? Find out if mail ordering during the summer is safe.

CL Answer
Do hair loss supplements, such as Viviscal, Hair La Vie, and Nutrafol, or topical essential oils work?
Vitamins and supplements that may help with hair loss and thinning, including saw palmetto, beta-sitosterol, protein, iron, and vitamin D.

CL Answer
What are phytoceramides? Do phytoceramide supplements really work to improve aging skin?
Phytoceramide supplement information, including what they are, what they claim to do, and if they have anti-aging effects.

Recalls & Warnings
April 09, 2003
Recall of Dangerous Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On April 4, 2003, the U.S. Food and Drug Administration's MedWatch program announced that Ultra Health Laboratories, Inc. and Bionate International, Inc. are warning consumers not to purchase or consume a product known as Vinarol tablets.
Recalls & Warnings
March 20, 2020
Seller of Rejuvenation Pills Settles Charges of Making False Claims
Health Center, Inc. has agreed to halt their allegedly deceptive advertising claims about three "cure-all" health and wellness products that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 10, 2024
StellaLife Homeopathic Oral Rinse & Spray Recalled Due to Yeast, Mold, and Bacteria
On June 5, 2024, HomeoCare Laboratories, Inc.
Recalls & Warnings
July 16, 2024
Infla-650 Herbal Dietary Supplement Recalled
On July 16, 2024, Guru Inc. issued a recall of one lot of Infla-650 Herbal Dietary Capsules because they were found to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
December 10, 2024
Aroma Vita Hot Cocoa Mix Recalled Due to Metal Fragments
On October 2, 2024, Dyma Brands Inc. issued a recall of two lots of Aroma Vita Hot Cocoa Mix, because they may contain metal fragments which, in severe cases, could lead to damage of the digestive tract if ingested.
Recalls & Warnings
December 19, 2024
Jarritos Coconut Water Recalled
On November 4, 2024, Tipp Distributors, Inc. issued a recall all lot codes of Jarritos Coconut Water (17.5 fl oz cans) because the airtight seal on the lid of the cans may be compromised.
Recalls & Warnings
December 19, 2024
Force Forever Joint Pain Supplement Recalled
On December 12, 2024, GNMart INC recalled all lots of Force Forever, a supplement promoted for joint pain, because FDA tests found it to contain undeclared diclofenac and dexamethasone.
Recalls & Warnings
December 26, 2024
Sleep Supplement Recalled Due to Undeclared Soy
On October 30, 2024, Deseret Biologicals Inc. recalled 2,547 bottles of DesBio lunaSOMM Natural Sleep Support Dietary Supplement capsules, which are labeled as containing phosphatidylserine from sunflower lecithin powder but contain undeclared soy lecithin.
Recalls & Warnings
January 04, 2024
Lone Star Botanicals Warned by FDA for Manufacturing Violations
On November 26, 2023, the FDA issued a Warning Letter to Lone Star Botanicals, Inc.
News Release
February 01, 2012
Top-rated vitamin and supplement brands and merchants on consumer satisfaction for 2012 -- Based on ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,260 responses collected in November, 2011. Most respondents used multiple supplements.
Recalls & Warnings
October 15, 2012
Recall of Nutrition Bars Containing Peanut Butter Due To Salmonella Risk
On October 11 and 12, 2012, four brands of nutrition bars were voluntarily recalled due to possible salmonella contamination of the peanut butter used in each of these bars. The peanut butter was produced by Sunland Inc., and is part of a larger recall of Sunland's peanut butter and peanut products.
Recalls & Warnings
April 25, 2017
Beware of Products Which Promise to Treat or Cure Cancer, FDA Warns
On April 25, 2017, the FDA warned consumers to be aware of supplements and other products claiming to cure cancer.
Recalls & Warnings
May 19, 2020
FTC Sends Refund Checks to Consumers of Unproven Weight Loss and Sexual Enhancement Supplements
On May 19, 2020, the FTC announced it is mailing 143,636 checks totaling more than $8,500,000 to consumers who purchased deceptively marketed supplements.
Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
October 02, 2023
Dangerous Ingredients Found in Ten Arthritis and Pain Supplements
On September 29, 2023, the FDA warned consumers that the following arthritis and pain management products contain undeclared drugs such as diclofenac, methocarbamol (a muscle relaxant), and dexamethasone (a corticosteroid), and/or other potentially harmful ingredients.
Recalls & Warnings
December 14, 2023
FDA Warns Seller of Novid NASAL SPRAY for Allergy, Virus, and Cold Claims
On December 7, 2023, the FDA issued a warning to Novid Group, Inc. following an inspection of the company’s website, which found statements about the company’s novid NASAL SPRAY product to be drug claims.
Recalls & Warnings
March 11, 2024
Healthex Warned for Manufacturing Violations
On June 15, 2023, the FDA issued a Warning Letter to Healthex Distributors, Inc.
Recalls & Warnings
May 04, 2023
PrimeZEN Enhancement Supplement for Men Found to Contain Prescription Drug
On April 27, 2023, the FDA issued a warning letter to Tager Online, Inc. DBA Volt Candy; Volt Candy Wholesale Club after FDA laboratory analysis determined PrimeZEN Black 6000, a product promoted to improve sexual function for men, contains tadalafil and sildenafil.
Recalls & Warnings
April 03, 2023
SimplyProtein Bars Recalled Due to Undeclared Cashews
On March 31, 2023, Wellness Natural USA Inc. issued a voluntary recall of a single lot of SimplyProtein Peanut Butter Chocolate Crispy Bars because they contain undeclared trace of tree nuts (cashews).
Recalls & Warnings
April 24, 2023
Terry Naturally and EuroMedica B Vitamins Recalled Due to Allergen Risk
On April 21, 2023, EuroPharma, Inc. issued a voluntary recall of 4 lots of its Terry Naturally BioActive Vitamin B 60 count and 3 lots of its EuroMedica Active B Complex 60 count products due to undeclared milk. No illnesses have been reported to date.
Recalls & Warnings
July 26, 2023
Five Leaf Pet Botanicals Warned by FDA for Drug Claims
On June 22, 2023, the FDA issued a warning letter to Five Leaf Pet Botanicals, Inc.
Recalls & Warnings
June 14, 2023
Havasu Beet Root Powder Recalled Due to Allergen Risk
On June 12, 2023, Supplement Manufacturing Partner Inc. issued a recall of one lot of Havasu Nutrition’s Beet Root Powder + due to undeclared milk. The recall was initiated following a report of an allergic reaction from a consumer with a milk allergy.
Recalls & Warnings
May 29, 2021
FDA, FTC Warns Five Sellers of "Infertility" Supplements
The FDA and FTC (Federal Trade Commission) sent warning letters to the following five companies in May for illegally selling dietary supplements promoted with claims to treat infertility and other reproductive health issues:
Recalls & Warnings
March 09, 2022
FDA Warns Seller of Magnesium, CBD, Herbal Extracts & More
On February 9, 2022, the FDA issued a warning letter to Bea Lydecker’s Naturals, Inc.
Recalls & Warnings
August 01, 2022
53 Protein, Meal Replacement and Nutritional Beverages Recalled Due to Bacterial Contamination
On July 29, 2022, Lyons Magnus LLC issued a recall of 53 protein, meal replacement, and nutritional beverages, including those sold under the brand names of Glucerna, Premier Protein, Oatly, and others, due to the potential for microbial contamination, including from Cronobacter sakazakii.
Recalls & Warnings
May 08, 2024
Razer, Inc. to Pay Over $1.1 Million Settlement for Zephyr Face Mask ”N-95” Claims
On April 29, 2024, the FTC announced it will be returning over $1.1 million to consumers who purchased Zephyr face masks after the company promoted its Zephyr face masks as N95-grade despite never submitting for testing to the FDA for approval of such claim.
News Release
March 13, 2006
ConsumerLab.com reports on supplements for bone health containing calcium and vitamin D— Results posted for 32 supplements for adults and children; One found contaminated with lead
WESTCHESTER COUNTY, NEW YORK — MARCH 13, 2006 — ConsumerLab.com announced test results today from its new Product Review of Supplements for Bone Health covering 32 adult and children's products containing calcium and vitamin D. Sales of calcium supplements in the U.S.
Recalls & Warnings
November 21, 2022
Healthy Sense and People’s Choice Multis Recalled
On November 17, 2022, Mason Vitamins Inc.
Recalls & Warnings
December 01, 2022
FTC Takes Action Against Company Promoting “COVID Resist” Supplement to Treat COVID-19
On November 22, 2022, the FTC filed a complaint in a U.S. district court against California-based company Precision Patient Outcomes, Inc. for promoting its COVID Resist and VIRUS Resist supplements to prevent and treat COVID-19.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
March 23, 2023
FDA Warns Herbal Vitality for Promoting Supplements to Treat Cold & Flu, Kidney Stones & More
On March 7, 2023, the FDA issued a warning letter to Herbal Vitality, Inc.
Recalls & Warnings
June 08, 2022
Allergy Bee Nasal Swabs Recalled Due to Potential Contamination With Yeast, Mold & Bacteria
On June 7, 2022, Buzzagogo, Inc. issued a nationwide recall of one lot of Allergy Bee Gone for Kids Nasal Swab Remedy after FDA testing revealed elevated levels of yeast and mold. The agency also warned the recalled swabs may contain the bacteria Bacillus cereus.
Recalls & Warnings
June 02, 2022
Prune & Senna Softgels Recalled Due to Undeclared Peanuts
On March 26, 2022, Indiana Botanic Gardens Inc. issued a voluntary recall of one lot of Botanic Choice brand Prune & Senna Softgels because the product contained undeclared peanuts.
Recalls & Warnings
January 18, 2002
Recall of Pepsin-containing Digestive Supplements
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 16, 2002 Enforcement Report.
Recalls & Warnings
June 04, 2004
FTC Charges Marketers of Two Supplements with False Claims to Cure Range of Diseases
On June 3, 2004 the Federal Trade Commission (FTC) charged marketers of two dietary supplements with falsely claiming that their products can prevent and cure cancer and other diseases. According to the FTC’s complaint, Boston-area marketers Direct Marketing Concepts, Inc. (DMC), ITV Direct, Inc.
Recalls & Warnings
February 29, 2008
FTC Sues Sellers of Weight-Loss Pills for False Advertising
On February 8, 2008 the The Federal Trade Commission (FTC) charged a business operation with violating federal law by falsely claiming that its weight-loss pills cause users to lose weight without dieting or exercise.
Recalls & Warnings
December 27, 2019
Federal Court Shuts Down Three Supplement Companies With Serious Manufacturing Violations
Update: (1/23/20) ABH has issued a recall of all its products, which are sold under various brand names by over 800 distributors and retailers. More details are available in CL's post about this recall.
Recalls & Warnings
November 26, 2019
FDA Warns Companies Selling CBD Products as Dietary Supplements
On November 25, 2019, the FDA issued warning letters to 15 companies for selling products containing CBD (cannabidiol) labeled and marketed as dietary supplements, and/or for making drug claims about these products.
Recalls & Warnings
April 16, 2019
DMHA and Phenibut Are Not Permitted in Dietary Supplements, Warns FDA
On April 16, 2019, the FDA announced it has issued 11 warning letters to companies whose dietary supplement products contain the drugs DMHA or phenibut, and therefore are in violation of the law.
Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
FTC Warns 45 More Companies for Coronavirus Claims
On May 7, 2020, the FTC announced that it sent warning letters to 45 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 12, 2023
FDA Warns Company for Promoting Kratom Products for Pain, Mood, and More
On July 3, 2023, the FDA issued a warning letter to Sunshine Trading Company, Inc.
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
News Release
April 27, 2005
ConsumerLab.com finds quality of most nutrition powders and drinks high, but identifies five protein powders with excess sodium or cholesterol — Report Now Available Online
WHITE PLAINS, NY — APRIL 27, 2005 — ConsumerLab.com has released its latest report on the quality of nutrition powders, shakes and drinks. These products are used for general nutrition, dieting, and meal-replacement and are frequently used by athletes and bodybuilders.
Recalls & Warnings
January 19, 2022
FDA Warns Seller of Carnitine and Alpha Lipoic Acid Supplements
On November 18, 2021, the FDA issued a warning letter to GeroNova Research Inc.
Recalls & Warnings
January 12, 2022
FDA Warns Consumers Not to Use LuSys Laboratories COVID Tests Due to False Results
Update: (03/09/22) On February 17, 2022, the FDA issued a warning letter to LuSys Laboratories, Inc.
Recalls & Warnings
January 03, 2022
Seller of Wormwood, Aloe Supplement Warned by FDA
On December 7, 2021, the FDA issued a warning letter for Western Herb Products, Inc.
Recalls & Warnings
May 14, 2021
Maker of Care by Design CBD Settles Charges of Unsupported Claims
On May 5, 2021, Cannacraft, Inc.
Recalls & Warnings
May 10, 2021
Collagen & Biotin Supplement Recalled Due to Allergen Risk
On May 6, 2021, Bloommy, Inc. issued a recall of Bloommy Biotin Collagen Keratin Capsules for Skin, Joint, and Hair due to the presence of a fish allergen for the collagen-based ingredient.
Recalls & Warnings
May 08, 2021
FDA Warns Seller of Vitamins B12, C, D, and K
On April 7, 2021, the FDA issued a warning letter to Unived Inc following a review of the company's websites, which found statements made about the company's products Unived brand Colox, CalDveg, B12+D3, and D3+K2-7 to be drug claims.
Recalls & Warnings
July 10, 2021
FDA Warns Seller of Omega XL
On June 31, 2021 the FDA issued a warning letter to Great Healthworks, Inc. following a review of the company's website, which found statements made about Omega XL and ProbioticXL supplements to be drug claims.
Recalls & Warnings
June 22, 2021
Living Free Vitamins, Joint, Nerve and Other Supplements Recalled
On June 21, 2021, Bea Lydecker's Naturals, Inc. issued a recall of six Living Free brand supplements because the labels do not declare soy lecithin.
Recalls & Warnings
June 22, 2021
Prairie Wolf Hand Sanitizer Recalled
On June 21, 2021, Prairie Wolf Spirits, Inc. recalled all lots of Prairie Wolf Distillery hand sanitizer packaged in 16.9 fluid ounce and 20 fluid ounce containers that resemble water bottles because the products pose a risk of ingestion.
Recalls & Warnings
November 24, 2021
FDA Warns Sanitizer Corporation For Manufacturing and Misbranding Violations
On September 24, 2021, the FDA issued a warning letter to Chameleon Beverage Co. Inc.
Recalls & Warnings
February 10, 2022
Three Sexual Enhancement Supplements Sold on Amazon Recalled
On February 8, 2022, specific lots of three male sexual enhancement supplements, Red Mammoth capsules, MAC DADDY RED capsules, MAC DADDY PURPLE capsules, and The Red Pill capsules, were recalled because Amazon laboratory analyses found them to contain undeclared ...
Recalls & Warnings
May 01, 2021
Hand Sanitizer Sold at Ulta, TJ Maxx, and Marshalls Recalled Due to Toxic Chemicals
On April 28, 2021, Scentsational Soaps & Candles, Inc.
Recalls & Warnings
May 09, 2022
FDA Warns Five Companies for Selling CBD Supplements, Gummies and Creams With Delta-8 THC
On May 4th, 2022, the FDA issued warning letters to five companies for selling CBD and other products labeled as containing delta-8 tetrahydrocannabinol (delta-8 THC).
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
Recalls & Warnings
April 06, 2022
Mandalorian and Mickey Mouse Hand Sanitizers Recalled Due to Benzene, Methanol
On April 1, 2022, Best Brands Consumer Products, Inc.
Recalls & Warnings
February 24, 2023
Bountiful Charged with “Review Hijacking,” False Advertising of Nature’s Bounty Supplements on Amazon
On February 16, 2023, the Federal Trade Commission (FTC) charged The Bountiful Company (formerly NBTY), whose brands include Nature’s Bounty and Sundown, with deceptively using Amazon reviews and badges (such as “#1 Best Seller” and “Amazon’s Choice”) from its ...
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
April 23, 2019
"Brain Boosting" Supplements Were Promoted With Non-Existent Clinical Studies
On April 10th, 2019, the FTC (Federal Trade Commission) announced the marketers of cognitive enhancement supplements Geniux, Xcel, EVO, and Ion-Z have agreed to settle charges that they made false claims about the product, including fake research references and celebrity ...
Recalls & Warnings
October 12, 2005
Supplement Company to Pay $2 Million Penalty For Alleged Violations of FTC Order
On October 12, 2005 the Federal Trade Commission (FTC) announced that under the terms of a consent decree approved by the it for submission by the U.S. Department of Justice (DOJ) to the federal court for approval, NBTY, Inc. (NBTY, formerly Nature’s Bounty, Inc.
Recalls & Warnings
April 19, 2007
Recall of Another Sex Enhancement Supplement
On April 17, 2007 the Food and Drug Administration (FDA) website posted an announcement that Jen-On Herbal Science International, Inc. is conducting a voluntary nationwide recall of the company's supplement product sold under the name H S Joy of Love. Jen-On Herbal Science International, Inc.
Recalls & Warnings
November 15, 2009
Nationwide Recall of a Weight Loss Supplement Found to Contain Undeclared Drug Ingredients
On November 12, 2009, The U.S. Food and Drug Administration (FDA) announced that it informed GMP Herbal Products, Inc. that its product "Pai You Guo," a weight loss dietary supplement, contains undeclared drug ingredients.
Recalls & Warnings
December 17, 2004
FDA Seizes Ginseng Because of Potentially Risky Pesticide Residues
On December 16, 2004, the Food and Drug Administration (FDA) announced that the U.S. District Court for the District of New Jersey issued a warrant for the seizure of imported ginseng held for sale at FCC Products, Inc., located in Livingston, N.J. The U.S.
Recalls & Warnings
July 01, 2003
Direct Marketers of Weight Loss, Impotence, and Arthritis Supplements Charged with Deceptive Claims
On July 1, 2003, the Federal Trade Commission (FTC) announced three enforcement actions against direct marketers of weight-loss products containing ephedra. The two settlements and one complaint, filed in U.S.
Recalls & Warnings
August 11, 2015
Company Ordered to Stop Making and Selling Supplements
On August 4, 2015, a federal court ordered a permanent injunction against dietary supplement company Atrium Inc., and two companies under the same ownerhship, Aspen Group Inc., Nutri-Pak of Wisconsin Inc.
Recalls & Warnings
February 01, 2018
Seller of Supplements for Opiate Withdrawal Warned for Drug Claims
On January 11, 2018, the FDA sent warning letters to ten sellers of supplements promoted to treat opiate withdrawal following reviews of the companies' websites and social media which found statements and testimonials made about the products to be drug claims.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
March 16, 2021
FDA Warns Seller of Essential Oils
On March 12, 2021, the FDA issued a warning letter to Ravenscroft Apothecary, Inc.
Recalls & Warnings
November 10, 2020
FDA Warns Seller of Digestive Enzymes, Nattokinase, and More
On October 27, 2020, the FDA issued a warning letter to World Nutrition, Inc.
Recalls & Warnings
November 02, 2020
Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 30, 2020, the FDA issued a warning letter to Spartan Enterprises Inc. dba Watershed Wellness Center for selling the silver product Dissolve BioActive Silicate with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
October 15, 2020
FDA Warns Five Sellers of Dangerous Cesium Salt Supplements
On October 9, the FDA issued warning letters to five companies for selling products containing cesium chloride. The FDA has previously warned consumers not to use dietary supplements containing cesium chloride or any other cesium salt.
Recalls & Warnings
February 22, 2021
FDA Warns Sellers of St. John's Wort
On February 18, 2021, the FDA issued warning letters to two companies following reviews of the companies' websites, which found statements made about the companies' St. John's Wort products to be drug claims. These products include St.
Recalls & Warnings
February 16, 2021
Seller of BioCBD+ Products Warned for COVID-19 and Drug Claims
On February 11, 2021, the FDA issued a warning letter to Evolved Ayurvedic Discoveries, Inc.
Recalls & Warnings
February 02, 2021
FDA Warns Seller of Reishi Mushroom Supplements
On January 6, 2021, the FDA issued a warning letter to Entia Biosciences, Inc following a review of the company's website, which found statements made about the company's products Lion's Mane - All Natural Supplement, Antrodia Mushroom Powder, Maitake Mushroom Powder, Reishi ...
Recalls & Warnings
January 31, 2021
Protein and Fiber Bars Recalled Due to Allergen Risk
On January 28, 2021, think! and Interpac Technologies, Inc. issued a voluntary recall of think! Protein + Fiber Oatmeal: Farmer's Market Berry Crumble due to the potential presence of tree nuts, including almonds and pecans.
Recalls & Warnings
January 20, 2021
FDA Warns Seller of Vitamin D, Curcumin and CoQ10 Promoted for Treating COVID-19
On December 28, 2020, the FDA issued a warning letter to Smarter Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Smarter Curcumin, Smarter Ubiquinol, and Smarter Vitamin D3 to be drug claims.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Joint and Hair Loss Products
On December 22, 2020, the FDA issued a warning letter to Speedwinds Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Synotrex and Sephren to be drug claims.
Recalls & Warnings
June 30, 2020
FDA Warns Seller of Alpha Lipoic Acid, Berberine, and More
On June 18, 2020, the FDA issued a warning letter to Only Natural, Inc. dba Bio Nutrition, Inc.
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
Recalls & Warnings
June 09, 2020
Six More Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On June 5, 2020, the FTC announced that it sent warning letters to six multi-level marketing companies for selling products such as immune system boosters and probiotics with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrepresenting potential earnings people who have ...
Recalls & Warnings
September 11, 2020
Seller of "COVID PACK" Warned for Coronavirus Claims
On September 9, 2020, the FDA issued a warning letter to Pharmacy Plus, Inc. dba Vital Care Compounder for selling COVID PACK and COVID 'POSITIVE' PACK products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 08, 2020
Seller of "Diabetes Supplement" & More Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Nutritional Supplements Corporation Inc following a review of the company's website, which found statements made about some of the company's products, including Vitadone, Diabrex, and Viadevita to be drug claims.
Recalls & Warnings
September 08, 2020
FDA Warns Seller of Multivitamin for Manufacturing Violations
On August 14, 2020, the FDA issued a warning letter to Revival Products, Inc.
Recalls & Warnings
August 13, 2020
FDA Warns Three More Companies Selling Unapproved COVID-19 Tests
Between July 23 and 24, the FDA issued warning letters to three companies for marketing unapproved, adulterated or misbranded antibody tests for coronavirus (COVID-19).
Recalls & Warnings
April 21, 2011
FTC Targets Fake News Sites Making Deceptive Acai Claims
On April 19, 2011, the Federal Trade Commission (FTC) requested federal courts to temporarily halt the allegedly deceptive tactics of 10 operations using fake news websites to market acai berry weight-loss products.
Recalls & Warnings
August 02, 2012
FDA Warns Two Supplement Makers of Manufacturing Violations
On July 19, 2012, the FDA issued a warning letter to H & L Jerch Sales, Inc. for failing to maintain written quality control procedures, which constitutes a violation of current Good Manufacturing Practices (cGMP) and caused the company’s products to be declared adulterated.
Recalls & Warnings
July 05, 2012
FDA Warns Good Herbs Inc. of Manufacturing Violations and Drug Claims
The FDA issued a warning letter to Good Herbs Inc. on May 24, 2012, after a facility inspection found the company to be in violation of current manufacturing regulations, including lack of documentation and written procedures.
Recalls & Warnings
January 10, 2013
FDA Warns Aloe and Herbal Supplement Companies For Manufacturing Violations
AloeScience Labs, Inc. -- On November 14, 2012, the FDA issued a warning letter to AloeScience Labs, Inc.
Recalls & Warnings
March 16, 2023
Omega-3 Supplements for Dogs and Cats Recalled Due to Potential for Vitamin A Toxicity
On March 9, 2023, Stratford Care USA issued a recall of 62 brands of omega-3 supplements for dogs and cats due to potentially elevated levels of vitamin A.
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
March 19, 2004
Seasilver Stopped from Making Claim to Cure "Over 650" Diseases
On March 17, the Food and Drug Administration (FDA) announced that Seasilver USA, Inc., and Americaloe, Inc.
Recalls & Warnings
April 01, 2004
Aloe Producer Recalls Product Due to Toxic Levels of Vitamin D
On March 26.2004, the Food and Drug Administration (FDA) announced that Aloe Commodities International, Inc., Carrollton, Texas, is recalling 1600 bottles of Solutions IE Ageless Formula II, Lot numbers P2207 and P2221 because they contain a significantly higher-than-labeled level of vitamin D3.
Recalls & Warnings
October 18, 2005
FTC Stops False Claims about HGH Oral Spray
On October 18, 2005, the Federal Trade Commission (FTC) announced that, at its request, a federal court issued a temporary restraining order against marketers of oral sprays that supposedly contain human growth hormone (HGH) to stop them from making alleged false and deceptive claims and from ...
Recalls & Warnings
August 31, 2009
Court Orders Marketers of Supreme Greens and Coral Calcium to Pay Nearly $70 Million for Consumer Refunds
On August 27, 2009, a federal district court ordered the marketers of two dietary supplements – "Supreme Greens" and "Coral Calcium" – who claimed the products would cure ailments ranging from cancer and Parkinson’s disease to heart disease and autoimmune diseases to pay nearly $70 million for ...
Recalls & Warnings
October 06, 2011
Testosterone Booster Supplement Recalled for Containing Synthetic Steroid
On October 5, 2011, the U.S. FDA announced that Superior Metabolic Technologies Inc of Marietta, Georgia is voluntarily recalling all lots of the testosterone booster Uprizing 2.
Recalls & Warnings
January 02, 2012
FDA Warns XYMOGEN of Manufacturing and Labeling Violations Regarding Multiple Products
On December 13, 2011, the U.S. FDA sent a Warning Letter to Atlantic Pro Nutrients, Inc. (dba XYMOGEN) regarding labeling and/or manufacturing violations relating to its products, which include Borage CP-240, CoQmax CF, Immune Rx, Iron Glycinate, and Green Tea 600.
Recalls & Warnings
October 30, 2014
Seller of Vitamin C, Iron and Detox Supplements Warned for Violations, Drug Claims
On October 16, 2014, the FDA issued a warning letter to Vitalab Co., Inc. (which manufactures and labels supplements for three distributors: V.E. Irons, Inc., Springreen Products, Inc., and Sonne's Organic Foods, Inc.
Recalls & Warnings
January 23, 2020
Massive Recall of Supplements Sold by Over 800 Brands
On January 21, 2020, a group of contract manufacturers of dietary supplements issued a recall affecting products sold by over 800 brands that sell the products under their own brand names and labels.
Recalls & Warnings
December 24, 2020
Wash-Free Hand Sanitizer With Potentially Toxic Ingredient Recalled
On December 23, 2020, Shane Erickson, Inc.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
Recalls & Warnings
October 10, 2024
Seaweed Recalled Due to Allergen Risk
On October 10, 2024, Wismettac Asian Foods, Inc. recalled its 3.52 oz packages of Shirakiku Korean Seasoned Seaweed.
Recalls & Warnings
January 13, 2025
NuGo Nutrition Bar Recall Expanded
On January 10, 2025, Lifestyle Evolution Inc. expanded its recall of NuGo Dark Chocolate Chip nutrition bars to include NuGo Dark Chocolate Pretzel nutrition bars and nutrition bar multipacks, due to an undeclared milk allergen.
Recalls & Warnings
February 05, 2025
Muscle Tech Alpha Test Recalled
On December 18, 2024, Iovate Health Sciences USA Inc. recalled 10 lots (163,248 units) of Muscle Tech Alpha Test capsules because they contain cathine, a controlled substance that can cause serious adverse effects.
Recalls & Warnings
February 20, 2025
Maker of Zicam Agrees to Pay $6 Million to Settle Lawsuit Over Claims to “Shorten Colds”
The maker of Zicam Cold Remedy products, Church & Dwight Co., Inc., has agreed to pay up to $6 million to settle a class action lawsuit that charged it with promoting the products to reduce the severity of cold symptoms and reduce the duration of colds without reliable scientific evidence.
Recalls & Warnings
February 24, 2025
Vitality Sexual Enhancement Supplement Recalled
On February 20, 2025, One Source Nutrition recalled all lots of Vitality capsules after FDA analysis found the supplements to contain undeclared prescription drugs sildenafil and tadalafil, which are not permitted in dietary supplements.
Recalls & Warnings
May 09, 2024
DaVinci Laboratories Memory Supplement Recalled
On May 8, 2024, DaVinci Laboratories issued a recall of one lot (approximately 72 bottles) of Amyloid Complete capsules because they may contain undeclared shellfish (shrimp and crab).
Recalls & Warnings
April 25, 2024
Yogi Tea Recalled Due to Pesticides
On April 19, 2024, Yogi Tea issued a recall of over 54,800 packs of Yogi Echinacea Immune Support tea after a sample of echinacea tested above acceptable limits for multiple pesticides. No illnesses have been reported to date.
Recalls & Warnings
April 29, 2024
Longreen Reishi and Xlim Express Coffee Recalled Due to Undeclared Allergens
On April 18, 2024, BF Suma Pharmaceuticals, Inc.
Recalls & Warnings
June 13, 2024
Thyroid Supplement Recalled Due to Salmonella
On June 11, 2024, Penn Herb Company Ltd issued a recall of 51 bottles of Nature’s Wonderland Thyroid Formula capsules after FDA testing revealed the products to be contaminated with Salmonella.
Recalls & Warnings
April 17, 2015
Herbal Laxative and "Detox" Kit Containing High Levels of Lead and/or Arsenic Recalled in Canada
On April 15, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that St. Francis Herb Farm Inc.
Recalls & Warnings
May 20, 2014
FTC Charges Seller of Green Coffee Bean with False Weight Loss Claims, Fake Websites
On May 15, 2014, the Federal Trade Commission (FTC) filed a lawsuit against the sellers of Pure Green Coffee for making unsubstantiated weight loss claims and deceiving consumers with fake "news" websites.
Recalls & Warnings
January 09, 2014
Marketers of "Genetically Customized" Supplements Settle FTC Charges of Deceptive Health Claims
Two marketers of "genetically customized" nutritional supplements have agreed to a settlement with the Federal Trade Commission (FTC) over charges that the companies made deceptive advertising claims and did not adequately protect customers' medical and financial information.
Recalls & Warnings
October 17, 2013
Turmeric Spice Powder Recalled Due To High Lead Levels
On October 3, 2013, the OnTime Distribution Inc. issued a voluntary recall of PRAN brand spice powder Turmeric because it was found to contain high levels of lead.
Recalls & Warnings
November 30, 2023
Original The Rock Capsules Recalled Due to Undeclared Sildenafil
On October 18, 2023, Noah’s Wholesale, LLC issued a nationwide recall of one lot of the company’s Original The Rock capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
May 08, 2023
SD Biosensor Pilot At-Home COVID-19 Tests Recalled Due to Bacterial Contamination
On May 4, 2023, the FDA warned consumers and health care providers not to use certain lots of Roche Diagnostics’ SD Biosensor, Inc. Pilot COVID-19 At-Home Tests due to bacterial contamination in the test kit’s liquid solution.
Recalls & Warnings
August 25, 2023
FDA Warns Consumers Not to Use Big Guys Male Energy Supplement
On August 22, 2023, the FDA warned consumers not to buy or use BIG GUYS Male Energy Supplement after FDA laboratory analysis found it to contain undeclared sildenafil.
Recalls & Warnings
February 19, 2019
Alaskan Smoked Silver Salmon in Jars and Cans Recalled Due to Risk of Botulism
On February 15, 2019, Smoked Alaska Seafoods, Inc. recalled all jars and cans of Smoked Silver Salmon because it has the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life-threatening illness or death.
News Release
May 28, 2004
ConsumerLab.com warns of deceptive campaign by manufacturer of children's vitamin
WHITE PLAINS, NY — FOR IMMEDIATE RELEASE — FRIDAY MAY 28, 2004 — Responding to a deceptive public relations campaign recently launched by vitamin manufacturer Northwest Natural Products, Inc., ConsumerLab.
News Release
December 30, 2002
Top quality melatonin products identified by ConsumerLab.com — Hormone supplement used to treat sleep disturbances due to jet travel and other causes
WHITE PLAINS, NY — December 30, 2002 — In its latest Product Review, ConsumerLab.com found that 16 of 18 melatonin dietary supplements met their label claims and were free of lead contamination.
News Release
October 29, 2002
Enormous variation found in strength of garlic supplements — most popular herb in U.S. — ConsumerLab.com releases results online today
WHITE PLAINS, NY — October 29, 2002 — ConsumerLab.com's Product Review of Garlic Supplements found strength to vary by as much as 1500% across products. Strength was based on each product's ability to generate allicin, a chemical associated with the efficacy of non-aged garlic.
News Release
March 05, 2002
ConsumerLab.com finds nutrition powders & drinks more accurately labeled than nutrition bars, but unapproved food ingredient seen in some
White Plains, NY -- March 5, 2002 — ConsumerLab.com announced today that 24 of 26 nutrition powders and drinks that it recently evaluated met their label claims for carbohydrates, fats, and proteins. This is in stark contrast to ConsumerLab.
Recalls & Warnings
April 26, 2015
FDA Identifies More Products Listing Synthetic Amphetamine
On April 22, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called beta- methylphenylethylamine (BMPEA) on product labels.
Recalls & Warnings
December 05, 2015
FDA Warns Companies Selling Products Containing Picamilon
On November 30, 2015, the FDA issued warning letters to five supplement companies selling products containing picamilon, which is not a lawful dietary supplement ingredient in the U.S.
Recalls & Warnings
January 19, 2002
Recall of Certain Iron Supplements
The U.S. Food and Drug Administration (FDA) released the following Class III recall information in its January 16, 2002 Enforcement Report. A Class III recall is a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences:
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
January 24, 2003
FTC Challenges Weight-loss Claims for Slim Down Solution
The Federal Trade Commission today charged Slim Down Solution, LLC, Maderia Management, Inc., and several related companies and individuals with using false and unsubstantiated claims in the marketing and advertising of "Slim Down Solution" - a purported weight-loss product.
Recalls & Warnings
June 01, 2012
Homeopathic Manufacturer Settles False Advertising Suit
June 1, 2012 — A federal court in California recently authorized the settlement of a class action lawsuit against Boiron Inc. and Boiron USA, Inc.
Recalls & Warnings
October 05, 2011
FDA Warns Nutrition Center Inc (Nutri-West) of Manufacturing Violations
The FDA published a Warning Letter dated September 9, 2011 to Nutrition Center Inc. (dba Nutri-West) informing the firm of manufacturing violations found during an inspection of the firm's facility in Douglas, Wyoming in April, 2011.
Recalls & Warnings
September 08, 2011
FDA Warns Several Supplement Manufacturers Not Following Good Manufacturing Practices
In recent weeks, the U.S. FDA has sent warning letters to several supplement manufacturers regarding deficiencies identified during audits of their facilities and not adequately corrected. Use the links below to access the Warning Letters on the FDA website.
Recalls & Warnings
September 13, 2010
Supplement for "Increasing Desire" Recalled for Containing Drug
The U.S. FDA posted a notice regarding the recall of Masxtreme Capsules by the distributor, Natural Wellness, Inc. FDA analysis has determined the product contains undeclared amounts of Aminotadalafil, an analog of tadalafil.
Recalls & Warnings
July 20, 2010
Recall of ED Supplement Containing Drug
On July 20, 2010, the U.S. FDA posted a news announcement from Good Health, Inc. regarding the recall of Vialipro, a sexual enhancement supplement sold nationally.
Recalls & Warnings
October 05, 2007
FTC Charges Progesterone Cream Sellers with Making Unsubstantiated Claims
On October 5, 2007 the Federal Trade Commission (FTC) announced complaints against seven online sellers of alternative hormone replacement therapy (HRT) products, alleging that they made health claims for their natural progesterone creams without supporting scientific evidence.
Recalls & Warnings
September 25, 2007
Recall of Contaminated Baby Supplement
On September 21, 2007 the U.S. Food and Drug Administration (FDA) warned consumers not to consume Baby's Bliss Gripe Water, apple flavor, with a code of 26952V and expiration date of October 2008 (shown as '10/08' on the label). The product is distributed by MOM Enterprises, Inc.
Recalls & Warnings
August 09, 2007
FDA Warning on Red Yeast Rice Products
On August 9, 2007, the U.S. Food and Drug Administration (FDA) warned consumers not to buy or eat three red yeast rice products promoted and sold on Web sites. The products may contain an unauthorized drug that could be harmful to health.
Recalls & Warnings
June 14, 2007
Marketers of Bogus Growth Hormone Sprays Settle with FTC
On May 29, 2007 the Federal Trade Commission (FTC)announced that two operations that marketed oral sprays that were supposed to help users lose weight, reverse the aging process, and prevent or treat diseases have settled FTC charges that their claims were bogus.
Recalls & Warnings
December 27, 2012
Two Supplement Companies Warned For Manufacturing Violations
Sterling USA Neutraceutical Lab, LLC -- On November 16, 2012, the FDA issued a warning letter to Sterling USA Neutraceutical Lab, LLC following a facility inspection which found the company’s dietary supplements to be adulterated because they were have been prepared, packed, or held under ...
Recalls & Warnings
November 08, 2012
Chinese Herb Recalled Due To Undeclared Sulfites
On November 7, 2012, the New York State Department of Agriculture announced that Mayflower International Inc., aka Fu Xiang Yuan Trading Inc., is recalling Lily Dry after sample analysis revealed the product contains high levels of sulfites which were not declared on the label.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
August 24, 2012
Protein Shots, Drinks and Gelatin Recalled For Potential Botulism Contamination
On August 17, 2012, Protica Inc.
Recalls & Warnings
July 31, 2020
FDA Warns Seven Sellers of "Hangover Cures"
The FDA recently issued warning letters to seven companies for promoting hangover relief products with drug claims (use the links below to read the full warning letter):
Recalls & Warnings
June 23, 2020
BioPure Healing Products Warned for Manufacturing Violations
On June 17, 2020, the FDA issued a warning letter to BHP Holdings Inc.
Recalls & Warnings
August 14, 2020
The Green Herb and New Genesis Health Warned for Manufacturing Violations
On July 31, 2019, the FDA issued a warning letter to Davis Ventures, Inc.
Recalls & Warnings
July 07, 2020
Seller of Homeopathic Products Warned for Manufacturing Violations
On June 19, 2020, the FDA issued a warning letter to Washington Homeopathic Products, Inc.
Recalls & Warnings
July 28, 2022
FDA Warns Three Manufacturers of Sanitizer for Manufacturing and Misbranding Violations
Between July 8 and July 21, the FDA issued warning letters to three companies selling hand sanitizer products that violate Current Good Manufacturing Practices (cGMP), are adulterated and misbranded, and for refusal to allow the agency to access and copy company records.
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Recalls & Warnings
July 01, 2022
Male Enhancement Supplements Sold on Amazon Recalled
On January 27, 2022, Loud Muscle Science LLC issued a voluntary recall of various lots of Launch Sequence supplements because they were found to contain the prescription drug Tadalfil.
Recalls & Warnings
June 16, 2022
Omega 3 Supplements Recalled Due to Undeclared Drug Ingredients
On May 28, 2022, Latin Foods Market issued a voluntary recall of one lot of Artri King Reforzado con Ortiga y Omega 3 tablets after FDA laboratory analysis found the presence of undeclared diclofenac and dexamethasone.
Recalls & Warnings
January 12, 2023
FDA Warns Two Sellers Promoting CBD to Treat COVID-19
On January 10, 2023, the FDA issued warning letters to two companies following a review that found statements made on the companies’ websites to be drug claims because they promoted the cannabidiol (CBD) products to prevent or treat COVID-19.
Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Recalls & Warnings
November 21, 2022
6 Supplement Companies Warned by FDA for Making Cholesterol Claims
On May 4, 2022, the FDA issued warning letters to six supplement companies following review that found statements made on company websites and Walmart purchase pages suggesting the products could lower cholesterol to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
April 05, 2022
Sexual Enhancement Capsules for Men Recalled
On April 1, 2022, F&S Medical Supply, dba Pink Toyz, recalled one lot of Pink Pussycat 3000 mg capsules to the consumer level because FDA analysis found them to contain sildenafil.
Recalls & Warnings
February 22, 2024
Pacific BioLogic Co. Warned by FDA for Manufacturing Violations
On December 21, 2023, the FDA issued a Warning Letter to Curtis Jacquot, dba Pacific BioLogic Co.
Recalls & Warnings
January 04, 2007
Sellers of Popular Weight Loss Supplements Pay $25 Million Over FTC Allegations of Deceptive Advertising
On January 4, 2007, the Federal Trade Commission (FTC) announced that it had filed complaints in four separate cases alleging that weight-loss and weight-control claims were not supported by competent and reliable scientific evidence.
Recalls & Warnings
September 23, 2005
FTC Continues Litigation Against Makers of CortiSlim and CortiStress Supplements
On September 21, 2005 the Federal Trade Commission (FTC) announced that three defendants will give up $4.
Recalls & Warnings
September 15, 2005
Weight Loss Pill Marketer in Settlement with New Jersey Attorney General
On August 16, 2005, New Jersey Attorney General Peter C. Harvey and Consumer Affairs Director, Kimberly Ricketts, announced that Goen Technologies Corp.
Recalls & Warnings
May 28, 2003
FTC Challenges Claims of Five Supplements to Treat or Cure Serious Diseases
On May 27, 2003 the Federal Trade Commission announced that it had filed a complaint in federal district court against A.
Recalls & Warnings
March 05, 2020
Protein Powder Recalled Due to Undeclared Milk
On March 3, 2020, New Capstone, Inc. issued a recall of ReStructure Vanilla Protein Powder because it may contain undeclared milk.
Recalls & Warnings
November 05, 2021
Herb Recalled Due to Risk of Lead, Cadmium Contamination
On November 2, 2021, Murray Int'l Trading issued a recall of Herbal Doctor brand Angelicae Sinensis due to potential risk it may contain elevated levels of lead and cadmium.
Recalls & Warnings
May 04, 2021
CytoSport's Evolve Protein Shakes Recalled
On May 1, 2021, CytoSport, Inc., recalled certain lots of Evolve Protein Shakes because they have the potential to be contaminated with soy protein.
Recalls & Warnings
December 01, 2021
Probiotics Recalled Due to Bacterial Contamination, May Be Life-Threatening to Some
On December 1, 2021, Livia Global, Inc issued a voluntary recall of two lots of probiotics due to potential contamination with Pseudomonas aeruginosa. This bacterium, if ingested, can cause life-threatening infection in immunocompromised individuals.
Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
Recalls & Warnings
November 23, 2022
CBD Not Permitted in Gummies, Candies, Cookies, Candy, or Pet Treats, Says FDA
On November 16, 2022, the FDA issued warning letters to five companies for selling products such as gummies, tea, cookies, lollipops, fruit snacks, hard candies, and pet treats containing cannabidiol (CBD) and/or delta-8 tetrahydrocannabinol (delta-8 THC) as conventional food products.
Recalls & Warnings
September 16, 2015
Weight And Enhancement Supplement Recall Expanded
On September 11, 2015, One Minute Miracle Inc. issued a recall of all lots the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
September 05, 2015
Powdered Caffeine Risky, FDA Warns
On August 27, 2015, the FDA issued a warning letter to five companies selling caffeine powder, warning that the powder "presents a significant or unreasonable risk of illness or injury under the conditions of use recommended or suggested in the labeling."
Recalls & Warnings
January 31, 2017
Vegetarian Protein Drink Recalled Due to Undeclared Milk
On January 28, 2017 NSE Products, Inc. (a subsidiary of Nu Skin Enterprises, Inc.) issued a recall of its vegetarian ageLOC TR90 Protein Boost protein drink because it contains undeclared milk.
Recalls & Warnings
March 31, 2018
Supplement Manufacturer Shut Down for Manufacturing Violations
On March 29, 2018, the FDA announced that U.S. District Court for the Eastern District of New York has entered a consent decree of permanent injunction with Riddhi USA, Inc. and Mohd M. Alam, president and owner of Riddhi USA, Inc., for selling adulterated and misbranded dietary supplements.
Recalls & Warnings
December 01, 2017
Nutra Labs Recalls Sexual Enhancement Supplements Containing Undeclared Drugs
On November 30, 2017, Nutra Labs Inc. recalled certain lots of its sexual enhancement supplements Bull 1800 mg Capsules and Chao Jimengnan 150 mg Tablets because they were found to contain undeclared sildenafil.
Recalls & Warnings
May 29, 2003
Recall and Warning for Another Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On May 23, Best Life International, in cooperation with the U.S. Food and Drug Administration, warned consumers not to purchase or consume the product known as Viga.
Recalls & Warnings
July 13, 2005
Recall of Multivitamins with Iron Lacking Child-Resistant Caps
On July 12, 2005, The U.S. Consumer Product Safety Commission in cooperation with NBTY, Inc. announced a voluntary recall of the following consumer product. Consumers should stop using recalled products immediately unless otherwise instructed.
Recalls & Warnings
July 24, 2006
Marketers of Seasilver Ordered to Pay Almost $120 Million
On July 24, 2006, the Federal Trade Commission (FTC) announced that the marketers of Seasilver, an alleged phony cure-all, have been ordered to pay almost $120 million for failing to comply with an earlier order requiring them to pay $3 million in consumer redress.
Recalls & Warnings
November 18, 2003
Supplement Promoted As Cancer Treatment Removed from Market
On November 10, 2003, the Food and Drug Administration (FDA) announced that NBTY, Inc., of Bohemia, N.Y.
Recalls & Warnings
March 20, 2007
Recall of Adulterated Sexual Enhancement Supplement
As posted on the FDA website, on March 15, 2007, the company Barodon SF of Los Angeles, CA announced that it is conducting a voluntary nationwide recall of the company's supplement product sold under the name V.MAX.
Recalls & Warnings
April 22, 2008
Court Upholds Order for Seasilver Marketers to Pay $120 Million
On April 16, 2008, the Federal Trade Commission (FTC) announced that the U.S.
Recalls & Warnings
March 06, 2009
"The Truth About Nutrition" Marketers Agree to Pay $3 Million to Settle Charges of Deceptive Advertising of Dietary Supplements and Devices
On March 6, 2008, the Federal Trade Commission (FTC) announced that marketers of dietary supplements and health-related devices have agreed to pay $3 million in consumer redress to settle FTC charges that they deceptively claimed their products treated or prevented a wide variety of serious ...
Recalls & Warnings
December 26, 2010
Four Probiotic Products in Canada May Pose Serious Health Risks to Those with Milk or Soy Allergies
On December 24, 2010, Health Canada (the Canadian health ministry) advised consumers with milk or soy allergies that four probiotic natural health products are being voluntarily recalled from the market because they are labelled as not containing dairy (milk) and/or soy but may contain trace ...
Recalls & Warnings
September 15, 2010
Recall of Testosterone-Boosting Supplement
On September 14, 2010, the FDA posted a notice that KiloSports is recalling Clomed (60 count bottles), a supplement with claims to "release gonadatropin," "elevate testosterone" and "promote spermatogenesis."
Recalls & Warnings
December 20, 2011
Organic Celery Seed Products Recalled Due to Potential Salmonella Contamination
On December 16, 2011, the U.S. FDA posted a notice from Swanson Health Products regarding the recall of Swanson Organic Celery Seed (Whole) which is packaged in plastic bottles with a net weight of 1.4 oz. (40 grams) because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
November 27, 2011
FDA Seeks Permanent Injunction Against Dietary Supplement Maker -- 400+ Products Affected
On November 23, 2011, the U.S. FDA took legal action against a dietary supplement maker and owner for substituting ingredients and products without noting the changes on the final product labels. The permanent injunction, filed on behalf of the FDA by the U.S.
Recalls & Warnings
June 20, 2013
Glucosamine Legal Settlement Over Labeling -- Many Products Covered
On May 16, 2013, a U.S. District Court preliminarily approved a settlement of class action cases alleging that certain claims made on the labeling of specific glucosamine products are false, deceptive, and misleading. No allegations related to safety or physical injury have been made.
Recalls & Warnings
March 02, 2013
FDA Finds Manufacturing Violations At NSF-Certified Supplement Facility
The FDA recently warned dietary supplement manufacturer Beehive Botanicals, Inc.
Recalls & Warnings
February 06, 2013
Ingredient Supplier Warned For Meal Replacement Product Misbranding and Adulteration
On October 4, 2012, the FDA issued a warning letter to dietary supplement ingredient supplier Raw Deal, Inc.
Recalls & Warnings
January 04, 2014
Beware of Supplements Claiming to Treat Brain Injury, FDA Warns
On December 31, 2013, the FDA warned consumers to avoid dietary supplements promoted to prevent, treat or cure concussions and other traumatic brain injuries (TBIs).
Recalls & Warnings
April 11, 2015
Acai Berry Marketers Who Tricked Consumers with Fake News Sites Ordered to Pay $16 Million
On April 6, 2015, the Federal Trade Commission (FTC) announced that a recent U.S. district court ruling will require marketing affiliates for LeanSpa's acai berry and "colon cleanse" weight loss products to pay $16 million in consumer redress.
Recalls & Warnings
December 02, 2014
Glucosamine Class-Action Settlement Reversed by Appeals Court
On November 19, 2014, an appeals court rejected a settlement of a class-action lawsuit that alleged label claims on glucosamine products manufactured or sold by Rexall Sundown, Inc., NBTY, Inc. or their affiliates, were false, deceptive or misleading.
Recalls & Warnings
June 25, 2020
"Anti-Viral" Supplement Recalled for Unsupported Coronavirus Claims
On June 23, 2020, Golden Nutrition Inc. issued a recall of four lots of Anti-Viral Immune Enhancement Capsules because the label makes unsupported health claims to "help fight corona virus and influenza.
Recalls & Warnings
June 23, 2020
Seller of CBD Tinctures and "Immune Boost Packs" Warned for Coronavirus Claims
On June 18, 2020, the FDA issued a warning letter to Project 1600 Inc. for selling CBD products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 31, 2020
FTC Sues Golden Sunrise for Marketing Deceptive $23,000 Coronavirus Cure
On July 31, 2020, the Federal Trade Commission (FTC) charged Golden Sunrise Nutraceutical, Inc., a California-based company, with deceptively advertising a $23,000 treatment plan as a scientifically proven way to treat COVID-19.
Recalls & Warnings
July 17, 2020
Two More Hand Sanitizers That May Contain Toxic Ingredient Recalled
Update: (8/10/20) Soluciones Cosméticas has recalled additional products that may contain methanol, as listed in red below.
Recalls & Warnings
July 10, 2020
FTC Charges Seller With Falsely Promising Consumers Next Day Shipping of Face Masks and Other PPE
On July 8, 2020, the Federal Trade Commission (FTC) charged the online marketer SuperGoodDeals.com, Inc. and its owner, Kevin J. Lipsitz, with falsely promising consumers next-day shipping of facemasks and other personal protective equipment (PPE) to use during the coronavirus pandemic.
Recalls & Warnings
September 01, 2020
Harmonic Nature Hand Sanitizer Recalled
On August 28, 2020, Harmonic Nature recalled their hand sanitizer because it may contain 1-propanol, which is toxic when ingested.
Recalls & Warnings
March 04, 2021
Federal Court Bars Confidence USA from Selling Supplements
On March 4, 2021, the FDA announced a permanent injunction has been entered, barring dietary supplement manufacturer Confidence USA Inc. and two of its executives from producing or selling products until they become compliant with current good manufacturing practices (cGMPs).
Recalls & Warnings
December 11, 2020
Goldenseal Recalled Due to Microbial Contamination
On December 4, 2020, WishGarden Herbs, Inc. issued a recall of 14 lots of Cord Care Powder and Goldenseal Powder because they were manufactured using ingredients that were potentially contaminated with the bacterium Cronobacter sakazakii.
Recalls & Warnings
May 12, 2020
Plum Dragon Herbs Warned for Coronavirus Claims
On May 8, 2020, the FDA issued a warning letter to Plum Dragon Herbs, Inc. for selling Chinese medicine products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 24, 2021
Metal Reported in Church & Dwight Gummy Melatonin, Multis & Fiber Supplements
On April 20, 2021, Church & Dwight Co., Inc.
Recalls & Warnings
August 30, 2015
Weight and Enhancement Supplements Recalled
On August 27, 2015, One Minute Miracle Inc. issued a recall of one lot each the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
August 01, 2013
Fat Loss and Muscle Enhancement Supplements Found To Contain DMAA
On June 17, 2013, the FDA issued a warning letter to Formulife, Inc., dba Purus Labs, Inc., following a facility inspection which found the dietary supplements Fat Smack XR Thermolipolytic, Muscle Marinade Fresh Fruit and Muscle Marinade Cherry Limeade to contain DMAA.
Recalls & Warnings
April 04, 2013
Three More Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On April 3, 2013, the FDA warned consumers that the sexual enhancement supplements AFFIRM XL, Love Rider and Ninja Mojo were found to contain undeclared erectile dysfunction drugs. Consumers are urged to stop using these supplements immediately.
Recalls & Warnings
March 29, 2013
Prescription Drugs Found In Prostate and Sexual Enhancement Supplements
October 24, 2012 the FDA issued a warning letter to USA Far Ocean Group Inc./ Health & Beauty Group Inc. because a number of the company's dietary supplements were found to contain pharmaceutical drugs, or were promoted with statements that constitute drug claims.
Recalls & Warnings
October 24, 2012
FDA Seizes Many Supplements from New York Company Due To Drug Claims
On October 23, 2012, U.S. Marshalls, acting on behalf of the FDA, seized dietary supplements and unapproved drugs from Confidence, Inc., a supplement manufacturer in Port Washington, N.Y. The products included dietary supplements Dr.
Recalls & Warnings
December 21, 2012
FDA Warns Maker of Glucosamine and Omega-3 Supplements For Manufacturing Violations
On December 7, 2012, the FDA issued a warning letter to Basic Organics, Inc. following a facility inspection which found the company's Glucosamine Chondroitin and Omega 3 dietary supplements to be adulterated.
Recalls & Warnings
October 26, 2011
FDA Warns Supplement Maker of Manufacturing Violations Affecting Many Products
The U.S. FDA has sent a Warning Letter (dated 10/17/2011) to Health Advances USA, Inc. regarding manufacturing violations that cause its dietary supplement products to be considered adulterated.
Recalls & Warnings
June 21, 2011
FDA Seizes Probiotics Over Marketing Claims
On June 7, 2011, the U.S. FDA announced that, at its request, U.S. Marshals seized probiotic products from UAS Laboratories, Inc., of Eden Prairie, Minn. because the company markets the products as drugs.
Recalls & Warnings
July 15, 2010
Supplement Company Pays $5.5 Million to Settle False Advertising Claims
On July 14, 2010, the Federal Trade Commission (FTC), as part of its ongoing efforts to stop bogus health claims, announced that it is requiring a major marketer of dietary supplements to pay $5.
Recalls & Warnings
January 10, 2012
Acai Berry Pill Marketers to Pay $1.5 Million to Settle FTC Charges
On January 9, 2012, the U.S. Federal Trade Commission (FTC) announced that an operation that marketed acai berry supplements, "colon cleansers," and other products using allegedly fraudulent free trial offers and phony endorsements from Oprah Winfrey and Rachael Ray will pay $1.
Recalls & Warnings
May 01, 2009
FDA Warns Consumers to Stop Using Hydroxycut -- Product Being Tested by ConsumerLab.com
On May 1, 2009, the U.S. Food and Drug Administration warned consumers to immediately stop using Hydroxycut products by Iovate Health Sciences Inc., of Oakville, Ontario and distributed by Iovate Health Sciences USA Inc. of Blasdell, N.Y.
Recalls & Warnings
October 06, 2004
False Claims Made by Marketer of Cortisol-related Weight-Loss Supplements According to FTC
On October 6, 2004, the Federal Trade Commission (FTC)charged marketers of two dietary supplements with claiming, falsely and without substantiation, that their products can cause weight loss and reduce the risk of, or prevent, serious health conditions.
Recalls & Warnings
March 02, 2005
Marketer of Weight-Loss System Settles FTC Charges of Deceptive Advertising
On March 1, 2005, the Federal Trade Commission (FTC) announced that California infomercial producer Modern Interactive Technology, Inc.
Recalls & Warnings
January 13, 2019
Sexual Enhancement Supplement Recalled
On January 8, 2019, Happy Together, Inc. issued a recall of the sexual enhancement supplement Rhino 5k because FDA analysis found it to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
Recalls & Warnings
September 25, 2018
FDA Warns Seller of Digestive Enzyme Supplements
On March 6, 2018, the FDA issued a warning letter to Uckele Health & Nutrition, Inc.
Recalls & Warnings
June 29, 2018
Prescript-Assist Probiotic Recalled
On June 29, 2018, LL's Magnetic Clay, Inc., issued a recall of certain lots of Prescript-Assist, a "broad spectrum probiotic and prebiotic" supplement, because they may contain undeclared almonds, crustaceans, dairy, casein, eggs, and peanuts.
Recalls & Warnings
July 06, 2018
Prescript-Assist Probiotic Recall Expanded
On July 6, 2018, LL's Magnetic Clay, Inc., expanded its original recall of Prescript-Assist, a "broad spectrum probiotic and prebiotic" supplement, because it may contain undeclared almonds, crustaceans, dairy, casein, eggs, and peanuts.
Recalls & Warnings
May 12, 2018
Seller of Maca Powder Warned for Drug Claims
On April 18, 2018, the FDA issued a warning letter to Herbs America, Inc. following review of the company's website, and the company's Maca Magic store on www.amazon.
Recalls & Warnings
April 14, 2018
Men's Sexual Enhancement Supplement Recalled
On April 12, 2018, AMA Wholesale Inc. recalled its sexual enhancement supplement Rhino 69 Extreme 50000 because it was found to contain undeclared tadalafil.
Recalls & Warnings
November 29, 2019
FDA Warns Seller of "All Natural" Treatment for Opioid Withdrawal, Migraines & More
On November 18, 2019, the FDA issued a warning letter to EPH Technologies, Inc., following a review of the company's websites, which found statements made about some of the company's products, including Detoxoplex, Sinople, and Migrenz, to be drug claims.
Recalls & Warnings
March 31, 2020
Bulletproof Warned for Manufacturing Violations
On March 20, 2020, the FDA issued a warning letter to Bulletproof 360, Inc.
Recalls & Warnings
March 24, 2020
Mislabeled Lindt Chocolate Bar Recalled
On March 19, 2020, Lindt & Sprüngli (USA) Inc. issued a recall of one lot of Lindt Excellence 85% Cocoa chocolate bars because they are wrapped in the wrong packaging.
Recalls & Warnings
February 26, 2020
Company Failed to Report Adverse Events Associated With Its Nutrition Shakes
On February 12, 2020, the FDA sent a warning letter to Market America Inc for failure to submit serious adverse event reports about two of its products, as required by federal regulation. The company received two reports following serious adverse events but did not submit the proper forms:
Recalls & Warnings
February 11, 2020
FTC Sues Two Companies Selling Bone Health and Joint Pain Supplements
On February 11, 2020, the Federal Trade Commission (FTC) announced that it sued two companies to stop them from continuing to make false claims about their bone and joint health products, Ostinol (ZyCal Bioceuticals) and StimTein (Excellent Marketing Results, Inc. (EMR)).
Recalls & Warnings
January 24, 2020
Fat-Burning, Energy Supplement Linked to Heart Trouble
A 33-year-old woman in Australia developed cardiac ischemia (a lack of blood flow to the heart) after taking the "fat burning" supplement Alpha Lean-7, according to a recent report in the Journal of Sports Sciences.
Recalls & Warnings
March 09, 2020
FDA Warns Sellers of Essential Oils, Colloidal Silver & Teas Promoted to Treat Coronavirus
On March 9, 2020, the FDA and FTC announced they have issued joint warning letters to seven companies for selling products such as essential oils, teas and colloidal silver with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 08, 2019
Seller of Tea, Colloidal Silver, Homeopathic Products Warned for Manufacturing Violations
On September 12, 2019, the FDA issued a warning letter to Herbal Healer Academy, Inc.
Recalls & Warnings
October 01, 2019
Lead and Arsenic Found in Multi Mineral & Vitamin Supplement
On September 30, 2019, Cellect Products Inc. and Oglethorpe Ltd. issued a recall of one lot of Cellect®, Essentials Factor® Multi Mineral & Vitamin Supplement Unflavored Powder Mix because it was found to contain unsafe levels of lead and arsenic.
Recalls & Warnings
September 21, 2019
Liquid Vitamin C for Men Recalled
On September 16, 2019, Fitoterapia USA Inc. issued a recall of Macho Artificial Passion Fruit Flavored Vitamin C Liquid Supplement, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
July 23, 2019
FDA Warns Seller of CBD Lotions, Patches, Tinctures and Pet Products
On July 22, 2019, the FDA issued a warning letter to Curaleaf, Inc.
Recalls & Warnings
July 16, 2019
FDA Warns Seller of Magnesium and CBD
On July 9, 2019, the FDA issued a warning letter to Ceba-Tek, Inc.
Recalls & Warnings
June 16, 2019
"Immune Support" Throat Sprays Recalled
On June 13 2019, APS BioGroup, Inc. issued a recall of four "immune support" throat sprays because they have the potential to be contaminated with Stenotrophomonas maltophilia, a bacteria that can cause respiratory infection, particularly in individuals with weakened immune systems.
Recalls & Warnings
May 25, 2019
Department of Justice Files Complaint to Shut Down Supplement Company
On May 23, 2019, the United States Department of Justice announced a complaint seeking a permanent injunction has been filed against dietary supplement marketers Helen Chian and Jim Chao, president and company manager of Confidence USA Inc.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 24, 2003
Court Closes the Doors on Company That Sold Weight Loss Supplement
On October 24, 2003, the Federal Trade Commission (FTC)announced that Mark Nutritionals, Inc. and Edward D’Alessandro, Jr. have agreed to settle federal charges that they used false and unsubstantiated claims to sell their weight-loss product.
Recalls & Warnings
March 17, 2004
Marketers of “Focus Factor” and “V-Factor” Fined for Advertising Claims
On March 17 , 2004 the Federal Trade Commission (FTC) reported that the marketers of “Focus Factor,” a dietary supplement that purports to improve concentration, and “V-Factor,” a supplement that purports to enhance sexual performance, have agreed to settle charges that they made numerous ...
Recalls & Warnings
March 09, 2004
FTC Stops False Advertising by Makers of Weight Loss and Arthritis “Cures”
On March 9, 2004, the Federal Trade Commission (FTC) announced that several direct mail marketers have agreed to pay $2.
Recalls & Warnings
January 17, 2006
FDA Warns Consumers about Brazilian Diet Pills Found to Contain Active Drug Ingredients
On January 13,2006, the U.S. Food and Drug Administration (FDA) warned consumers not to use two unapproved drug products that are being marketed as dietary supplements for weight loss.
Recalls & Warnings
August 14, 2008
Airborne to Pay $30 Million for Deceptive Advertising of Cold Remedy -- Refunds Available
On August 14, 2008, the Federal Trade Commission (FTC) announced that Airborne Health, Inc.
Recalls & Warnings
August 20, 2019
Seller of Liposomal Curcumin, Vitamin C & Melatonin Warned for Manufacturing Violations
On August 6, 2019, the FDA issued a warning letter to Let's Talk Health, Inc.
Recalls & Warnings
January 23, 2020
Flaxseed Salmonella Risk Prompts Recall
Update: (2/4/20) This recall has been expanded to include additional Nopalina flaxseed products, which are highlighted below.
Recalls & Warnings
May 23, 2016
Amino Acid Supplements Recalled
On May 18, 2016, Ultimate Nutrition Inc. issued a recall of its Amino Gold amino acid tablets and capsules because they contain undeclared milk in the form of hydrolyzed whey protein.
Recalls & Warnings
March 08, 2016
Maker of Calcium and Vitamin C Supplements Warned for Manufacturing Violations
On September 17, 2015, the FDA issued a warning letter to Raphah, Inc.
Recalls & Warnings
July 18, 2017
Oat Cereal Recalled Due to Listeria Risk
On July 18, 2017, Garden of Light, Inc., recalled Woodstock Organic Matcha Vanilla Oats because of potential contamination with Listeria monocytogenes.
Recalls & Warnings
June 18, 2016
Antioxidant Supplement Recalled Due to Allergen Risk
On June 17, 2016 GMJ Natural Products Inc. issued a recall of 32 fluid oz. jugs of Jugo Moringa Plus Antioxidant because they contain undeclared whey protein.
Recalls & Warnings
October 25, 2016
Seller of Liquid Aloe & Mineral Supplement Warned for Manufacturing Violations
On August 10, 2016, the FDA issued a warning letter to Perfect Source Natural Products Inc.
Recalls & Warnings
September 23, 2017
Enhancement Supplement "Vegetable Vigra" Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled VEGETABLE VIGRA [sic] after FDA testing found undeclared presence of sildenafil.
Recalls & Warnings
September 21, 2017
Sexual Enhancement Supplements Containing Prescription Drugs Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled the following supplements because they were found to contain undeclared sildenafil and tadalafil:
- Rhino 7 Platinum 5000 capsules, Lot# R7-D5K1011H
Recalls & Warnings
July 11, 2017
Gluten Free Chocolate Chip Bars Recalled Due to Allergen Risk
On July 10, 2017, Coborn's, Inc. recalled packages of Gluten Free Chocolate Chip Bars because some were incorrectly labeled as Gluten Free Fudge Brownies.
Recalls & Warnings
July 04, 2017
FDA Warns Against Company's Unsafe Teething Tablets
On June 20, 2017, the FDA issued a warning letter to Raritan Pharmaceuticals, Inc. following a routine inspection of the facility when it found the company's Homeopathic Infant's Teething Tablets in violation of current good manufacturing practice (CGMP) regulations.
Recalls & Warnings
November 22, 2017
FDA Warns Distributor of "Testosterone Wellness for Men"
On November 8, 2017, the FDA sent a warning letter to Vita-Pure, Inc.
Recalls & Warnings
February 27, 2018
Testosterone, Thyroid, Other Supplements Recalled Due to Undeclared Milk
On February 23, 2018, Progressive Laboratories, Inc., issued a recall of the following four supplements because they may contain undeclared milk:
Recalls & Warnings
November 02, 2008
Sales of Weight-Loss Supplement Suspended -- May Contain Diuretic Drug
On October 31, 2008, Associated Press reported that makers of the weight loss supplement StarCaps have suspended sales following accusations that the product contains a non-listed pharmaceutical diuretic, Bumetanide.
Recalls & Warnings
July 23, 2009
Six Male Enhancement Supplements Found Adulterated
On July 15, 2009, the U.S. FDA announced that Obteron 1 Inc. dba Nature & Health Co. is recalling the following supplements: LibieXtreme, Y-4ever, Libimax X Liquid, Powermania Capsule, Powermania Liquid, and Herbal Disiac.
Recalls & Warnings
April 07, 2011
Prostate Drug Found in Prostate Supplement -- Recall Underway
On March 24, 2011 the U.S. FDA posted a voluntary recall notice for U-Prosta from USA Far Ocean Group, Inc.
Recalls & Warnings
October 07, 2011
Recall of Whey Protein Powders Due to Allergens
On October 6, 2010 the FDA posted a recall notice from Prolab Nutrition Inc. (dated September 30) indicating that some of its PROLAB protein powders are being recalled as they may contain undeclared milk, wheat and gluten allergens.
Recalls & Warnings
March 07, 2012
Melatonin in Cookies and Drinks -- Not Allowed, Says FDA
On December 8, 2011 FDA issued a letter to Revolt Distribution, Inc. warning that their food and beverage products contain an unsafe food additive.
Recalls & Warnings
January 31, 2012
FTC Stops Fake News Sites About Acai Berry Products
As published on the U.S. Federal Trade Commission's (FTC) website on January 25, 2011, six online marketers settled with the FTC to stop posting fake news on websites intended to promote acai berry supplements and other weight-loss products.
Recalls & Warnings
May 13, 2012
Caffeinated Water Illegally Promoted According to FDA
On April 24, 2012, the U.S. FDA sent a Warning Letter to Vitality Distributing, Inc. indicating that the company's product "avitae caffeinated water" is being illegally promoted as an unapproved new drug due to claims relating to its safety and efficacy, such as the following:
Recalls & Warnings
April 04, 2012
Maker of Protein Drinks and Supplements Warned of Manufacturing Violations
The FDA published a Warning Letter to Protica, Inc., a maker of protein drinks and supplements, regarding violations of manufacturing regulations discovered during inspection of its facility in Whitehall, PA. Affected products include the foods Amped Up-2oz, Fireball-2oz, Healthy Shot-2.
Recalls & Warnings
March 14, 2012
Manufacturing Violations by Maker of Lecithin, Soy Supplements
The FDA issued a Warning Letter (dated March 7, 2012) to Modern Products, Inc. regarding manufacturing violations in the production of the supplements Fearn Lecithin Granules, Fearn Liquid Lecithin, Fearn 100% Soy Protein Isolate, and Gayelord Hauser Brewers Yeast.
Recalls & Warnings
March 03, 2012
FDA Warns Vitaganic of Manufacturing Violations
On February 8, 2012, the U.S. FDA sent a Warning Letter to Vitaganic, Inc. regarding violations of Current Good Manufacturing Practic (CGMP) regulations for dietary supplements found during an inspection of it manufacturing facility in Sunnyvale, California in October 2011.
Recalls & Warnings
August 18, 2006
Restitution Program for Purchases of Lane Labs' Products
On August 17, 2006 the Food and Drug Administration (FDA) announced that it was notifying consumers of a restitution (refund) program for purchasers of three of Lane Labs-USA, Inc.'s products. The products are BeneFin, MGN-3 and SkinAnswer.
Recalls & Warnings
June 09, 2005
FTC Cracks Down on "HGH" Supplements; $20 Million in Consumer Redress
On June 9, 2005, The Federal Trade Commission (FTC) announced that two Florida businesses have agreed to a federal court order requiring them to pay up to $20 million in consumer redress – the largest monetary judgment ever obtained in an FTC health fraud case – to settle charges that they ...
Recalls & Warnings
March 04, 2003
U.S. Marshalls Seize Supplements Claiming to Prevent Cancer and Treat Arthritis
On February 12, 2003, at the request of the Food and Drug Administration (FDA), U.S. Marshals seized dietary supplement products from Global Source Management and Consulting, Inc., in Sunrise, Florida. U.S.
Recalls & Warnings
April 15, 2003
FTC Requires Scientific Evidence for "Snore Formula" Claims
On April 15 2003, the U.S. Federal Trade Commission (FTC) announced that an Arizona-based company, Snore Formula, Inc., its officers, and a distributor have agreed to settle FTC charges that they failed to have scientific substantiation for the claims made for "Dr.
Recalls & Warnings
January 18, 2003
Non-Prescription Anti-Hypertension Pills Recalled
On January 17, the U.S. FDA's MedWatch Program reported that Herbsland Inc.
Recalls & Warnings
December 11, 2002
Recall of Calcium Supplement Possibly Contaminated with Antibiotic
The U.S. Food and Drug Administration (FDA) released the following Class II recall information in its December 11, 2002 Enforcement Report.
Recalls & Warnings
June 30, 2002
Recall of Dalyvite Liquid Multivitamin
In its June 26, 2002 Enforcement Report, the U.S. FDA reported the following Class III Food Recall. A Class III recall is a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
Recalls & Warnings
January 16, 2013
Nutrition Bars Recalled Due To Undeclared Allergen
On January 15, 2012, Belmont Confections Inc. issued a voluntary recall of Dymatize Nutrition Elite Gourmet Cookies & Cream bars and Dymatize Nutrition Elite Gourmet Fudge Brownie bars because they may contain undeclared peanuts.
Recalls & Warnings
December 05, 2012
Hydroxycut Class Action Lawsuit To Be Settled
Iovate Health Sciences Inc. has agreed to a settlement of $25.3 million, pending final court approval, in a class action lawsuit that alleged the company deceived consumers about the safety of its Hydroxycut weight loss supplements.
Recalls & Warnings
November 28, 2012
Maker of Pycnogenol, Memory and Immune Supplements Warned For Manufacturing Violations and Misbranding
On November 15, 2012, the FDA issued a warning letter to contract manufacturer Health Technology, Inc.
Recalls & Warnings
October 17, 2012
Maker of Probiotic Supplement Warned for Manufacturing Violations
On September 28, 2012, the FDA issued a warning letter to dietary supplement manufacturer James G. Cole Inc.
Recalls & Warnings
November 14, 2012
Mushroom Supplement Manufacturer Warned For Drug Claims and Adulteration
On October 18, 2012, the FDA issued a warning letter to Mushroom Wisdom, Inc. following a facility inspection and product label review which found the dietary supplements Amyloban 3399 From Lion's Mane, Grifron Maitake, and Super Shiitake to be promoted as drugs.
Recalls & Warnings
June 13, 2012
Tart Cherry Supplement Selller Warned by FDA Over Illegal Health Claims
The U.S. FDA recently published a Warning Letter (dated June 8, 2012) to Michelle's Miracle, Inc. for illegally promoting a range of tart cherry dietary supplements using health claims reserved for drugs. The products cited are:
Recalls & Warnings
September 10, 2012
Maker of Concussion Recovery Supplement Warned For Making Drug Claims
On August 28, 2012, the FDA issued a warning letter to Trinity Sports Group, Inc. after a review of the company's websites found statements about Neuro Impact Concussion Response Formula constituted drug claims not permitted on supplements.
Recalls & Warnings
September 08, 2012
Medifast Settles False Advertising Suit
On September 7, 2012, Jason Pharmaceuticals Inc., a wholly owned subsidiary of Medifast, agreed to pay a $3.7 million civil penalty and meet new compliance requirements as part of a settlement with the U.S. Department of Justice and the Federal Trade Commission.
Recalls & Warnings
February 28, 2013
Maker of Liquid Omega-3 Supplements Warned For Manufacturing Violations
On February 20, 2013, the FDA issued a warning letter to Capco Custom Packaging Inc.
Recalls & Warnings
February 04, 2013
Protein Supplement Recalled Due To Undeclared Soy and Milk
On January 30, 2013, R-Kane Products, Inc. issued a recall of Z PRO HIGH PROTEIN SUPPLEMENT after an FDA inspection found the potential allergens soy and milk were not listed on individual packet labels of the supplement.
Recalls & Warnings
February 15, 2013
Energy Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of the energy supplement MET-Rx Extreme Thermo Rage Watermelon 8 fl. oz. (Manufactured by MET-Rx Nutrition, Inc.) due to bacterial contamination.
Recalls & Warnings
February 14, 2013
Protein Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of PURE PROTEIN PROTEIN SHOT 25g PROTEIN, GRAPE Flavor PROTEIN DRINK 2.9 fl. Oz. (Manufactured by Worldwide Sport Nutritional Supplements, Inc.) due to potential bacterial contamination.
Recalls & Warnings
June 12, 2013
Biotin Supplement Found To Contain Only 4% to 5% of Labeled Amount
On May 22, 2013, the FDA issued a warning letter to Metaugus, Inc., following a facility inspection which found the company’s BIOTIN 3,000 mcg to be misbranded. The product's label was found to be false and misleading, and did not provide information required by federal regulations.
Recalls & Warnings
May 03, 2013
Maker of Probiotic, Iron and Folic Acid Supplements Warned For Manufacturing Violations
On April 17, 2013, the FDA issued a warning letter to Hillestad Pharmaceuticals USA, Inc.
Recalls & Warnings
April 19, 2013
Recall: Contaminated Iron Supplement
On March 18, 2013, Magno Humphries Inc. issued a voluntary recall of Ferrous Sulfate Tablets 325 mg (5 Grain) , an iron dietary supplement, after the company discovered it was contaminated with small blue pieces of polypropylene, a kind of plastic.
Recalls & Warnings
August 28, 2013
Maker of Sexual Enhancement Supplement Warned for Manufacturing Violations
On July 11, 2013, the FDA issued a warning letter to Precise Nutrition International Inc.
Recalls & Warnings
October 25, 2013
Supplement Company Warned For Numerous Manufacturing Violations
On August 2, 2013, the FDA issued a warning letter to DNE Nutraceuticals, Inc., following a facility inspection which found the company's products to be adulterated because they were packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
March 21, 2014
Homeopathic Products Recalled Due to Allergy Risk
On March 20, 2014, Terra-Medica, Inc. issued a voluntary recall of 56 lots of homeopathic products including, Pleo-FORT, Pleo-QUENT, Pleo-NOT, Pleo-STOLO, Pleo-NOTA-QUENT, and Pleo-EX.
Recalls & Warnings
March 19, 2014
Maker of Herbal Capsules and Extracts Warned for Manufacturing Violations
On September 19, 2013, the FDA issued a warning letter to Herbalist and Alchemist, Inc.
Recalls & Warnings
July 15, 2014
Pinnacle Labs International Warned for Manufacturing Violations
On June 24, 2014, the FDA issued a warning letter to Pinnacle Labs International, Inc.
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
April 03, 2014
Maker of Green Coffee Bean Extract and Weight Loss Supplement Warned for Manufacturing Violations
On March 13, 2014, the FDA issued a warning letter to Libi Labs, Inc.
Recalls & Warnings
December 05, 2014
Maker of Immune Supplement Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Urban Moonshine, Inc.
Recalls & Warnings
July 01, 2014
Maker of Elderberry Concentrate Warned for Manufacturing Violations
On June 23, 2014, the FDA issued a warning letter to Wyldewood Cellars Inc.
Recalls & Warnings
April 06, 2018
Workout Supplements Recalled Due To Allergen Risk
On April 5, 2018, Independent Nutrition Inc, dba Back to Health of Eugene., issued a recall of certain lots of Ignite High Endurance Pre-Workout Supplements because they may contain undeclared milk.
Recalls & Warnings
July 03, 2018
FDA Warns Seller of Adulterated Chinese Herbal Supplements
On June 20, 2018, the FDA issued a warning letter to KPC Products, Inc.
Recalls & Warnings
June 19, 2018
FDA Warns Seller of Weight Control Patches
On June 6, 2018, the FDA issued a warning letter to Health Management Group, Inc.
Recalls & Warnings
June 05, 2018
FDA Warns Seller of Colloidal Silver
On May 17, 2018, the FDA issued a warning letter to Silver Armor, Inc.
Recalls & Warnings
May 22, 2018
FDA Warns Companies Selling "Sun Protection" Supplements
On May 18, 2018, the FDA issued warning letters to four companies selling supplements for sun protection because statements made on product labels, websites and in marketing materials were found to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
May 19, 2018
Homeopathic Teething Drops, Nausea Drops, Silver-Zinc Throat Spray & More Recalled
On May 18, 2018, MBI Distributing, Inc.
Recalls & Warnings
May 19, 2018
Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On May 17, 2018, Shoreside Enterprises, Inc., recalled certain lots of its sexual enhancement supplements 7K and Poseidon 4500 (Extreme 1000 mg) because they were found to contain undeclared sildenafil and/or tadalafil.
Recalls & Warnings
May 15, 2018
Seller of Apple Cider Vinegar, Joint Supplements & More Warned for Drug Claims
On April 25, 2018, the FDA issued a warning letter to Baker's Best Health Products, Inc.
Recalls & Warnings
September 22, 2018
MyoWhey Protein Powder Recalled
On September 20, 2018, Purus Labs, Inc. issued a recall of one lot of MyoWhey Chocolate Cookie Crunch protein powder because it may contain undeclared milk and soy.
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
September 01, 2018
Homeopathic Remedies Contaminated With Bacteria
On August 29, 2018, Hellolife, Inc.
Recalls & Warnings
December 12, 2018
Seller of Protein Powder, Spirulina & Other Products Warned for Manufacturing Violations
On October 31, 2018, the FDA issued a warning letter to DynaPro International, Inc.
Recalls & Warnings
December 01, 2018
Seller of 5-HTP, Potassium & More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to The Delano Company, Inc.
Recalls & Warnings
November 27, 2018
More Dog Food Recalled Due to Risk of Vitamin D Toxicity
On November 27, 2018, Sunshine Mills, Inc. issued a recall of select Evolve Puppy, Sportsman's Pride Large Breed Puppy and Triumph Chicken and Rice Dog Food because they may contain elevated levels of vitamin D.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
March 26, 2019
Seller of NeuSilver Colloidal Silver for Adults, Children and Pets Warned by FDA
On February 19, 2019, the FDA issued a warning letter to iMedDo, Inc., following a review of the company's website, http://www.imeddo.
Recalls & Warnings
March 23, 2019
BLUEFUSION Capsules for Sexual Enhancement Recalled
On March 21, 2019, Ata Int. Inc. issued a recall of its BLUEFUSION capsules, which are promoted for sexual enhancement, because FDA analysis found them to contain sildenafil, tadalafil, desmethyl carbodenafil, dithiodesmethyl carbodenafil, scutellarin and daidzein.
Recalls & Warnings
July 21, 2003
FTC Stops False Advertising of Heavily Promoted Supplements for Breast Enhancment, Male Virility, and Snoring
On June 10, 2003, the Federal Trade Commission (FTC) announced that infomercial marketers Wellquest International, Inc. and Tony Hoffman Productions, Inc.
Recalls & Warnings
August 31, 2004
Two Makers of Weight Loss and Sex Enhancement Supplements Stopped From Making Unsubstantiated Claims
On August 27, 2004, the Federal Trade Commmission (FTC) reported that two Maine-based dietary supplement marketers and their principals have agreed to settle FTC charges that they made deceptive advertising claims about their dietary supplement products, in violation of federal law.
Recalls & Warnings
September 12, 2018
FDA Urges Consumers to Avoid Kratom
On September 11, 2018, FDA Commissioner Scott Gottlieb, M.D., urged consumers not to use products containing the kratom, an herb that is often promoted for pain relief and for relieving symptoms of opioid withdrawal.
Recalls & Warnings
September 08, 2018
Homeopathic Oral Sprays Recalled Due to Possible Bacterial Contamination
On September 5, 2018, Beaumont Bio Med, Inc., issued a recall of all of its water and alcohol-based homeopathic products because they have the potential to contain microbial contaminants.
Recalls & Warnings
February 17, 2018
FDA Warns Seller Reishi Mushroom Supplements
On February 7, 2018, the FDA issued a warning letter to Reishi D. International, Inc., following a facility inspection which found the company's Reishi D.
Recalls & Warnings
March 16, 2018
FTC Sends Refund Checks to Consumers of Unproven Weight-Loss Products
On March 15, 2018, the FTC announced it is mailing 18,301 checks totaling more than $437,000 to consumers who purchased weight-loss products from Colby Fox, Christopher Reinhold, and their companies, Tachht, Inc. and Teqqi, LLC.
Recalls & Warnings
January 30, 2018
Limbrel for Osteoarthritis Recalled Due to Risk of Adverse Effects
On January 26, 2018, Primus Pharmaceuticals, Inc. of Scottsdale, Arizona issued a recall of all unexpired lots of Limbrel products.
Recalls & Warnings
January 29, 2018
Seller of Male Enhancement, Prostate Supplements & More Warned for Drug Claims
On January 10, 2018, the FDA issues a warning letter to USA Labs AKA Power Source Distributors, Inc following a review of the company's website which found statements made about its products, including Maximum Male, Beta 300 (Beta Prosturol), Chromium Max 1000, DHEA, Healthy Cold-X and ...
Recalls & Warnings
January 26, 2018
Seller of Coenzyme-A Warned For Making Drug Claims
On January 5, 2018, the FDA issued a warning letter to Coenzyme A, Inc. following a facility inspection and review of the company's website that found the company made drug claims about its product Coenzyme-A.
Recalls & Warnings
January 25, 2018
Bulletproof Collagen Protein Recalled
On January 25, 2018, Bulletproof 360, Inc issued a recall of its Bulletproof Collagen Protein supplement because it contains undeclared milk.
Recalls & Warnings
December 26, 2017
Seller of Fish Oil, Vitamin D, Whey Protein and More Warned for Manufacturing Violations
On December 19, 2017, the FDA issued a warning letter to Maine Natural Health, Inc.
Recalls & Warnings
December 23, 2017
Seller of Meal Replacement, Protein Drinks, Cranberry and More Warned for Manufacturing Violations
On July 6, 2017, the FDA issued a warning letter to Professional Botanicals, Inc.
Recalls & Warnings
November 12, 2017
Weight Loss and Cleanse Supplements Contain Adulterated and Improperly Labeled Ingredients, FDA Warns
On November 2, 2017, the FDA issued a warning letter to Create-A-Pack Foods, Inc.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
October 17, 2017
Organic Tarragon Spice Recalled Due to Salmonella Risk
On October 16, 2017, Organic Spices, Inc. dba Spicely Organics issued a recall of its Organic Tarragon because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
October 13, 2017
Hi-Tech To Pay Over 40 Million to Settle FTC Charges of False Claims
On October 11, 2017, a U.S. District Judge ruled that Hi-Tech Pharmaceuticals Inc.
Recalls & Warnings
September 28, 2017
Seller of Prostate, Reishi Supplements Warned for Manufacturing Violations
On September 11, 2017, the FDA issued a warning letter to Vicare International (USA), Inc.
Recalls & Warnings
September 26, 2017
FDA Warns Seller of DHEA, Creatine, Chromium & More for Manufacturing Violations, Drug Claims
On August 25, 2017, the FDA issued a warning letter to Mega-Pro Nutrition, Inc., following inspections of the facility and website www.mega-pro.
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
June 17, 2017
Protein Bars and Bites Recalled Due to Listeria Risk
On June 15, 2017, Bulletproof 360, Inc. recalled five Collagen Protein Bar and Bite products because of potential contamination with Listeria monocytogenes.
Recalls & Warnings
June 10, 2017
Seller of Prostate, Immune Supplements & More Warned for Manufacturing Violations
On May 25, 2017 the FDA issued a warning letter to The Herbalist, Inc.
Recalls & Warnings
May 30, 2017
Maker of Collagen Supplement Warned for Manufacturing Violations
On July 17, 2014, the FDA issued a warning letter to Morhaim Pharmalab, Inc.
Recalls & Warnings
May 02, 2017
Seller of Amino Acid Supplements & More Warned for Manufacturing Violations
On April 20, 2017 the FDA issued a warning letter to Naturecom Inc.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
July 24, 2017
Protein Bars Recalled Due to Listeria Risk
On July 21, 2017, Bhu Foods recalled a number of their products after their organic sunflower seeds, distributed by Hudson Valley Foods, Inc., were potentially contaminated with Listeria monoocytogenes.
Recalls & Warnings
April 19, 2017
Sexual Enhancement Supplements for Men and Women Recalled
On April 18, 2017, Organic Herbal Supply, Inc. issued a recall of all lots of the following sexual enhancement supplements for men because they were found to contain the undeclared drug tadalafil:
Recalls & Warnings
April 04, 2017
Seller of B Vitamins, Vitamin C, Potassium & More Warned for Manufacturing Violations
On March 24, 2017 the FDA issued a warning letter to The Sanapac Company, Inc.
Recalls & Warnings
March 21, 2017
Twenty-one Sexual Enhancement Products Recalled
On March 7, 2017, A&H Focal Inc. issued a recall of the following sexual enhancement supplements because they were found to contain drugs such as sildenafil, tadalafil, vardenafil:
Recalls & Warnings
February 21, 2017
Sexual Enhancement Supplement Recalled
On February 16, 2017, Organic Herbal Supply, Inc. issued a recall of its sexual enhancement supplement XtraHRD Natural Male Enhancement because it was found to contain tadalafil.
Recalls & Warnings
February 16, 2017
U.S. Department of Justice Files Permanent Injunction Against Supplement Manufacturer
On February 16, 2017, the United States Department of Justice filed a complaint against Pick and Pay, Inc.
Recalls & Warnings
February 14, 2017
Life Extension Warned for Drug Claims
On February 1, 2017, the FDA issued a warning letter to Life Extension Foundation Buyers Club, Inc.
Recalls & Warnings
February 09, 2017
Herbal Supplement Found to Contain Ephedra Alkaloids Recalled
On February 7, 2017, Kingsway Trading Inc. issued a recall of Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains ephedra alkaloids.
Recalls & Warnings
February 09, 2017
Federal Court Orders Dietary Supplement Distributor to Stop Selling Its Products
On February 9, 2017, the FDA announced that VivaCeuticals Inc, doing business as Regeneca Worldwide, has been ordered by a federal court to stop selling its products, which were found to contain unsafe ingredients including DMAA.
Recalls & Warnings
February 07, 2017
Seller of Chinese Herbal Products for Prostate, Menopause and More Warned for Manufacturing Violations
On January 23, 2017, the FDA issued a warning letter to Herbal Sciences International, Inc.
Recalls & Warnings
October 18, 2016
Seller of Sublingual Vitamins Warned for Drug Claims
On August 31, 2016, the FDA issued a warning letter to Bio-Stasis International, Inc., following a review of the company's website which found statements made about its sublingual tablets, ViraPress, Vitamin D-3 and Vitamin B-12. to be drug claims.
Recalls & Warnings
October 08, 2016
Seller of Cell Power and Super Silica Warned for Manufacturing Violations, Misbranding
On September 23, 2016, the FDA issued a warning letter to SSO, Inc.
Recalls & Warnings
October 04, 2016
Turmeric Spice Recalled Due to Elevated Lead Levels
On September 26, 2016, Spices USA Inc. issued a recall of 772 bags of TASTY SAWA GROUND TURMERIC because it contains elevated levels of lead.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
September 02, 2016
Maker of Prescription Multi to Pay $15.5 Million to Settle Lawsuit Over Ingredient Claim
Endo Health Solutions Inc. has reportedly agreed to pay $15.5 million to settle a class action lawsuit which alleged its Qualitest Multi-Vitamin with Fluoride Chewable Tablets contained just 44% of the fluoride claimed on the label.
Recalls & Warnings
August 31, 2016
Seller of Joint Supplement Warned for Manufacturing Violations, Drug Claims
On July 15, 2016, the FDA issued a warning letter to Vitalife Inc.
Recalls & Warnings
August 23, 2016
Seller of Aloe Liquid and Capsules Warned for Manufacturing Violations, Drug Claims
On July 15, 2016, the FDA issued a warning letter to Aloe Farms, Inc.
Recalls & Warnings
August 08, 2016
Turmeric Spice Recall Expanded to Include More Brands
On August 5, 2016, Gel Spice, Inc. expanded its recall of one lot of Fresh Finds Ground Turmeric Powder to include other turmeric spices sold under different brand names due to elevated levels of lead.
Recalls & Warnings
August 05, 2016
More Turmeric Spice Recalled
On August 5, 2016, JM Exotic Foods, Inc. issued a recall of ground turmeric because it was found to contain elevated levels of lead.
Recalls & Warnings
July 29, 2016
Ground Turmeric Spice Recalled Due to Lead Contamination
On July 28, 2016, Gel Spice, Inc. issued a voluntary recall one lot of Fresh Finds Ground Turmeric Powder because it was found to contain elevated levels of lead.
Recalls & Warnings
July 23, 2016
Maker of "Super Food" Warned for Manufacturing Violations
On July 12, 2016, the FDA issued a warning letter to TerraVare, Inc.
Recalls & Warnings
July 16, 2016
Herbalife Agrees to Business Restructure and $200 Million Payment to Settle FTC Charges of Deception
Herbalife and its associated companies (Herbalife International America Inc., Herbalife , Ltd.) has agreed to restructure its U.S.
Recalls & Warnings
May 24, 2016
Seller of Joint Health, Omega-3 Supplements & More Warned for Manufacturing Violations, Drug Claims
On May 13, 2016, the FDA issued a warning letter to Rocky Fork Formulas, Inc.
Recalls & Warnings
July 18, 2017
FDA Warns Seller of "Quick Slim with pure Hoodia Gardonii" and "Diabalance Herbal Blood Sugar Balance"
On July 11, 2017, the FDA issued a warning letter to Black Seed Herb, Inc.
Recalls & Warnings
July 11, 2017
FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral Supplements
On June 27, 2017, the FDA issued a Warning Letter to Dr. Carolyn Dean of New Capstone, Inc.
Recalls & Warnings
January 21, 2017
Seller of "Cancer Kits," Alpha Lipoic Acid Supplements and More Warned for Drug Claims
On December 22, 2016, the FDA issued a warning letter to Northern Health Products, Inc. following a review of the company's websites, www.northernhealthproducts.com and www.petdca.
Recalls & Warnings
December 03, 2016
Male Enhancement Supplement Recalled
On November 29, 2016, MS Bionic, Inc. issued a recall of all lots of Megajex Natural Male Sex Enhancer capsules because they were found to contain tadalafil and sildenafil.
Recalls & Warnings
November 25, 2016
DMAA Product Recalled
On November 23, 2016, NutriVitaShop (a dba of Naturecom Inc.) issued a recall of its DMAA net weight 500g because it may contain DMAA.
Recalls & Warnings
November 19, 2016
GNC Women's Multi Recalled Due to Undeclared Allergen
On November 16, 2016, Nutra Manufacturing, Inc. issued a recall of one lot of GNC Women's Ultra Mega Time Release multivitamin because it contains undeclared milk.
Recalls & Warnings
February 23, 2016
Flax Seed and Chia Powders Recalled
On February 20, 2016, HEALTH MATTERS AMERICA INC., issued a recall of specific lots of Organic Traditions Sprouted Flax Seed Powder and Organic Traditions Sprouted Chia & Flax Seed Powder because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
February 06, 2016
Seller of Turmeric, Milk Thistle and More Warned for Manufacturing Violations, Drug Claims
On January 15, 2016, the FDA issued a warning letter to Terra Firma Botanicals, Inc.
Recalls & Warnings
February 03, 2016
Seller of Weight and Enhancement Supplements Warned For Drug Claims
On December 11, 2015, the FDA issued a warning letter to The One Minute Miracle, Inc., following a review of the company's website which found statements made about Miracle Diet 30 and Miracle Rock 48 to be drug claims.
Recalls & Warnings
January 26, 2016
Seller of CoQ10, SAM-e, Vitamin D, and More Warned for Manufacturing Violations, Drug Claims
On January 15, 2016, the FDA issued a warning letter to Nutri-Dyn Midwest, Inc., following a facility inspection which found the company's products, including Cardioauxin BP, Zinc Lozenge, Oliver, Pau D'Arco, Petadolex, Chondro Jointaide, Dynagesic, SAMe-200, Gugulipid.
Recalls & Warnings
January 21, 2016
Morphine Found in Licorice Product
On January 20, 2016, Master Herbs, Inc. issued a recall of all lots of Licorice Coughing Liquid because it was found to contain morphine.
Recalls & Warnings
January 20, 2016
Maker of Growth Hormone, Testosterone Booster Warned for Manufacturing Violations, Misbranding
On January 8, 2016, the FDA issued a warning letter to Nutraloid Labs Inc.
Recalls & Warnings
January 16, 2016
Seller of Liver, Lung Support Supplements Warned for Drug Claims
On January 4, 2016, the FDA issued a warning letter to Tibetan Herbal Balance, Inc.
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
December 12, 2015
Sexual Enhancement Supplement Recalled
On December 11, 2015, Reesna Inc. issued a recall of its sexual enhancement supplements Fuel Up Plus and Fuel Up High Octane which were distributed in August 2015 and were found to contain undeclared hydroxythiohomosildenafil.
Recalls & Warnings
May 15, 2016
Sexual Enhancement Supplements Recalled
On May 10, 2016, SOS Telecom, Inc. issued a recall of the following sexual enhancement supplements, which were found to contain undeclared sildenafil:
Recalls & Warnings
May 10, 2016
Multis and Energy Mixes Recalled
On May 9, 2016, Let's Talk Health, Inc. issued a recall of the following supplements because they contain the undeclared allergens soy and milk:
Recalls & Warnings
April 16, 2016
Workout Supplement Containing Acacia Rigidula Recalled
On March 18, 2016, Nubreed Nutrition, Inc. issued a recall of all lots of its "pre-workout" supplement Undisputed which is labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
April 06, 2016
FDA Warns of Stimulant Methylsynephrine In Supplements
On March 31, 2016, the FDA issued warning letters to seven companies selling products containing methylsynephrine (also called p-hydroxyephedrine or Oxilofrine), a stimulant drug which is not permitted in dietary supplements in the U.S.
Recalls & Warnings
March 29, 2016
Maker of Bone Health, Prostate, Cholesterol Supplements and More Warned for Drug Claims
On March 17, 2016, the FDA issued a warning letter to Rx Vitamins, Inc., following a facility inspection which found the company's products, includingTestost-Rx, DB-7, The Bone Density Formula, NaturLo Cholesterol, ThyRx-7 and Arth-9 to be misbranded.
Recalls & Warnings
March 15, 2016
FDA Warns Sellers of Weight and Workout Supplements Containing Acacia Rigidula
On March 7, 2016, the FDA issued warning letters to five sellers of supplements which were labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
November 11, 2015
Consuming a Single Energy Drink May Increase Cardiovascular Risk
A recently published study found that consumption of a single caffeinated energy drink significantly increased blood pressure and noradrenaline levels in healthy young adults. The researchers noted these changes could potentially increase the risk of cardiovascular events.
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
September 18, 2015
High Levels of Mercury and Lead Found in Ayurvedic Herbal Supplements
On September 17, 2015, the FDA announced that Shree Baidyanath Ayurved Bhawan Ayurvedic dietary supplements were found to contain high levels of lead and/or mercury, which can cause serious health problems.
Recalls & Warnings
August 22, 2015
Synthetic Amphetamine, Less Protein and More Sugar Than Claimed Found in Workout Supplements
On August 7, 2015, the FDA issued a warning letter to New Dawn Nutrition, Inc.
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
August 04, 2015
Whey, Casein and Colostrum Products Recalled
On August 3, 2015, Nutrition Resource Services, Inc. issued a voluntary recall of certain Just Be Natural (JBN) and Gifted Nutrition powders containing whey concentrate, whey isolate, casein, and colostrum because they contain undeclared milk.
Recalls & Warnings
July 28, 2015
Abnormal Heart Rhythms Linked with Unauthorized Natural Product, Health Canada Warns
On July 27, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that it has received a serious adverse reaction report of abnormal heart rhythms associated with the use of an unauthorized natural product in Canada called Remogen, which contains the drug ibogaine.
Recalls & Warnings
July 12, 2015
Marketers of Memory Supplement to Pay $1.4 Million to Settle FTC Charges
On July 8, 2015, the FTC announced the marketers of Procera AVH (Brain Research Labs Inc.) have agreed to pay $1.4 million to settle charges they made deceptive claims that the supplement could significantly improve memory, mood and cognitive function.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
May 26, 2015
Skin Measurement Device Used to Select Nutritional Supplements Not Approved for This Use, FDA Warns
On May 8, 2015, the FDA issued a warning letter to ZYTO Technologies, Inc.
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
May 16, 2015
FTC Mails Refund Checks to Nopalea Consumers
On May 15, 2015, the FTC announced it is mailing almost 500,000 checks totaling approximately $3 million to consumers who purchased the cactus-based fruit drink Nopalea (TriVita Inc).
Recalls & Warnings
May 13, 2015
Amberen Weight Loss Claims Not Supported by Evidence, Says FTC
On May 12, 2015, the FTC announced it filed a complaint to stop Lunada Biomedical Inc. from advertising that its product Amberen is clinically proven to cause substantial weight loss in women over 40.
Recalls & Warnings
April 29, 2015
FDA Targets Weight Loss and Workout Supplements Listing Synthetic Stimulant DMBA
On April 24, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called 1,3-dimethylbutylamine (DMBA) on product labels.
Recalls & Warnings
July 15, 2004
Company Stopped by FTC from Claiming to Prevent or Reverse Memory Loss without Evidence
On July 13, 2004, the Federal Trade Commission(FTC) announced that a dietary supplement manufacturer has settled FTC charges that it violated federal law by making unsubstantiated claims that its product, “Senior Moment,” could prevent memory loss and restore memory function in adults.
Recalls & Warnings
June 21, 2005
Marketers of “Himalayan Diet Breakthrough” Settle FTC Charges of Deceptive Advertising
On June 20, the Federal Trade Commission (FTC) announced that AVS Marketing, Inc., and its president, William R. Heid, have agreed to pay $400,000 in consumer redress to settle FTC charges that they deceptively marketed a purported weight-loss pill called “Himalayan Diet Breakthrough.
Recalls & Warnings
September 16, 2005
FTC Stops Weight-loss Claims about Seaweed-based Patches
On September 15, 2005, the U.S. Federal Trade Commission (FTC) announced that the alleged masterminds behind a fraudulent scheme to market two seaweed-based patches as weight-loss products to U.S. consumers have settled FTC charges.
Recalls & Warnings
October 15, 2007
US Marshalls Seize Charantea Supplements Promoted for Diabetes, Anemia and Hypertension
At the request of the U.S. Food and Drug Administration (FDA), on October 9, 2007, U.S. Marshals seized approximately $71,000 of goods from FulLife Natural Options, Inc., of Boca Raton, Fla., which marketed and distributed Charantea Ampalaya Capsules and Charantea Ampalaya Tea.
Recalls & Warnings
November 28, 2006
FTC Targets Seller of Pills Promoted for Height, Weight, and Bone Improvement
On November 28, 2006, the Federal Trade Commission (FTC) announced that a Florida business and its owner, who marketed purported height-enhancing pills for kids and young adults, will pay $375,000 to settle charges that their advertising claims were deceptive.
Recalls & Warnings
June 23, 2003
FDA Warns Consumers Against Taking 6 Sexual Enhancement Supplements
On June 20, 2003, the Food and Drug Administration (FDA) warned consumers not to purchase or consume the following products: SIGRA, STAMINA Rx and STAMINA Rx for Women, Y-Y, Spontane ES and Uroprin, manufactured by NVE, Inc., in Newton, N.J. and distributed by Hi-Tech in Norcross, Ga.
Recalls & Warnings
May 17, 2002
Update on Recall of Certain Ultra Slim-Fast with Soy Protein Drinks
In its May 15, 2002 Enforcement Report, the U.S. FDA reported the following Class III Food Recall. A Class III recall is a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
Recalls & Warnings
May 03, 2002
More Information on Recall of PC-SPES - Prostate Supplement
The FDA's May 1, 2002 Enforcement Report classified the recall of PC-SPES as a Class I recall. The recall was originally announced by its manufacturer in February with additional warnings from the FDA issued in March (see the 3/11/02 posting on ConsumerLab.
Recalls & Warnings
September 07, 2002
FDA Sends Warning Letters to Seven Online Supplement Sellers For Therapeutic Claims
On August 27, 2002, the FDA posted seven "Cyber" letters recently issued from its Center for Food Safety and Applied Nutrition (CFSAN) to internet website operators promoting dietary supplement products that claim to diagnose, mitigate, treat, cure, or prevent a specific disease or class of ...
Recalls & Warnings
March 02, 2012
Recall of Enhancement Supplement for Men -- Contains Drug
On Feb, 24, 2012, Regeneca, Inc. announced a voluntary nationwide recall of all lots of RegenErect. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a blue capsule sold individually in foil packets with a UPC code of 816860010055.
Recalls & Warnings
February 17, 2012
FDA Warns Supplement Company of Manufacturing Violations
The U.S. FDA recently posted a Warning Letter to Pure Encapsulations, Inc.
Recalls & Warnings
February 16, 2012
Recall of Enhancement Supplement for Women -- Contains Drug
On Feb, 10, 2012, Regeneca, Inc. announced a voluntary nationwide recall of RegenArouse Natural Female Intimacy Enhancement. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a pink capsule sold individually in foil packets.
Recalls & Warnings
February 11, 2012
Herbal Sprays for Colds and Cold Sores Recalled
On February 10, 2012 the U.S. FDA posted a notice announcing the recall of all "Koff & Kold" and "Kold Sore" sprays by Wholistic Herbs Inc. The products were distributed throughout the United States.
Recalls & Warnings
March 09, 2012
Maker of Inhaled Caffeine Product Gets Warning
The U.S. FDA issued a Warning Letter to Breathable Foods, Inc. (dated March 5, 2012) advising that their product AeroShot, which is promoted for "breathable energy," is falsely labeled (misbranded).
Recalls & Warnings
April 16, 2012
Protein Supplement Maker Fails FDA Audit -- Many Products Affected
The FDA sent a Warning Letter (dated April 2, 2012) to Theta Brothers Sports Nutrition, Inc. regarding numerous violations of good manufacturing practices at its Lakewood, New Jersey facility.
Recalls & Warnings
January 17, 2012
Recall of Calcium-Vitamin D Product That's Actually Glucosamine -- Allergy Alert
As published on the U.S. FDA's website, Rexall Inc. announced a recall on January 16, 2012 of Rexall Calcium 1200 mg plus 1000 IU Vitamin D3, 60 softgels. The affected bottles actually contain a TABLET product, Triple Strength Glucosamine Chondroitin and MSM Tablets.
Recalls & Warnings
March 03, 2012
Seller of Cancer Cures Warned by FDA
On February 9, 2012, the U.S. FDA sent a Warning Letter to BioAnue Laboratories, Inc. informing it that its websites at www.tumorx.com, www.cancerx.org, www.hopewelltechnologieslimited.com, and www.vmhe.
Recalls & Warnings
March 28, 2011
FDA Cracks Down on Violators of Supplement Manufacturing Rules
In February and March 2011, the U.S. FDA posted Warning Letters sent, respectively, to a distributor of dietary supplements and a supplement manufacturer for violations of current Good Manufacturing (GMP) rules.
Recalls & Warnings
February 24, 2011
Recall of Counterfeit Extenze Tablets Containing Drugs
On February 23, 2011, the FDA announced that Biotab Nutraceuticals, Inc. was notified that two lots of counterfeit product purporting to be Extenze contain undeclared drug ingredients. Specifically, lot 0709241 contains tadalafil and sildenafil, and lot 0509075 contains tadalafil and sibutramine.
Recalls & Warnings
September 13, 2010
ExtenZe Enhancement Supplements Seized in Canada
On August 19, 2010, Health Canada (Canada's health ministry) seized the sexual enhancement supplements "Male Enhancement ExtenZe" and "Women ExtenZe" which were imported from the U.S. Although legal and widely sold in the U.S.
Recalls & Warnings
October 08, 2010
FDA Warns of Stimulant in Slimming Capsules
On October 8, 2010, the U.S. FDA advised consumers who have Slimming Beauty Bitter Orange Slimming Capsules not to use the product. FDA warns that Slimming Beauty Bitter Orange Slimming Capsules contain the active pharmaceutical ingredient sibutramine, a prescription-only drug which is a stimulant.
Recalls & Warnings
June 21, 2011
FDA Warns Laboratory of Manufacturing Violations
On June 10, 2011, the U.S. FDA posted a Warning Letter (dated 4/15/11) to Nutro Laboratories, a division of NBTY, Inc., concerning serious violations of the current Good Manufacturing Practice (CGMP) regulations for Dietary Supplements based on its inspection of Nutro facilities in October 2010.
Recalls & Warnings
June 07, 2011
FDA Seizes Elderberry Juice Concentrate Due to Unproven Claims
On June 3, 2011, The U.S. FDA announced that, at its request, U.S. Marshals seized elderberry juice products that have been distributed by Wyldewood Cellars Inc., based in Peck, Kan., because the products are unapproved and misbranded drugs.
Recalls & Warnings
June 01, 2011
FDA Finds Pesticides in Ginseng Supplement
The U.S. FDA found pesticides in Root to Health, American Ginseng, Herbal Supplement (500 Veggie Capsules, 500 mg each) according to a May 13, 2011 Warning Letter from the FDA to the manufacturer of the product, Hsu's Ginseng Enterprises, Inc.
Recalls & Warnings
July 19, 2011
FDA Warns of Unsafe Drug in Slimming Supplements
On July 8, 2011, the U.S. FDA advised consumers not to purchase or use Slim Forte Slimming Capsule and Slim Forte Double Power Slimming Capsules. FDA laboratory analysis confirmed that these products contain sibutramine. Sibutramine is a controlled substance that was removed from the U.S.
Recalls & Warnings
October 05, 2011
FDA Warns Herbal Nitro of Manufacturing Violations
The FDA has published a Warning Letter dated September 22, 2011 to Herbal Nitro Inc. regarding manufacturing violations at its facility in Yucaipa, CA.
Recalls & Warnings
March 19, 2009
QVC Settles Charges of False Claims for Supplements
On March 19, 2009, the Federal Trade Commission (FTC) announced that QVC, Inc., a TV home shopping channel and one of the world’s largest multimedia retailers, has agreed to pay $7.
Recalls & Warnings
December 14, 2009
Nationwide Recall of Sexual Enhancement Supplements Containing Drug-like Compound
On December 14, 2009, the Federal Drug Administration (FDA) posted a notice on its website regarding the recall of many sexual enhancement supplements sold by Atlas Operations, Inc.
Recalls & Warnings
June 24, 2010
FDA Warns Seller of Pills Used to Treat Autism
On June 17, 2010 the U.S. Food and Drug Administration (FDA) sent a Warning Letter of legal violations to CTI Science, Inc., the marketer of OSR#1. The product is marketed as a supplement, but contains an ingredient that is not legally permitted to be sold as a supplement.
Recalls & Warnings
May 03, 2010
FDA Warns Consumers to Avoid Breath Supplement Contaminated with Lead
On May 3, 2010 the U.S. Food and Drug Administration warned consumers not to purchase nor consume Vita Breath, a dietary supplement manufactured by American Herbal Lab Inc. of Rosemead, Calif.
Recalls & Warnings
April 07, 2010
Nationwide Recall of Masxtreme Capsules Containing Drugs with Cardiovascular Side Effects
On March 30, 2010, the U.S. Food and Drug Administration (FDA) posted a notice from Natural Wellness warning consumers not to purchase or consume the product known as MasXtreme, Lot# 911035.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
August 28, 2008
FDA Finds Fault with Generic Toprol XL -- Problems Reported Earlier by ConsumerLab.com
On August 12, 2008, the U.S. FDA sent a letter to Sandoz Inc. warning of violations in its manufacture of Metoprolol Succinate ER tablets and other drug products. Sandoz's Metoprolol Succinate ER tablets are generic versions of Toprol XL.
Recalls & Warnings
March 08, 2007
FTC Files Against Maker of Calcium and Fertility Supplements
On January 29, 2007, the Federal Trade Commission (FTC) announced that it had filed civil contempt charges against Lane Labs, Inc.
Recalls & Warnings
May 17, 2007
Recall of Shark Cartilage Due to Potential Contamination
On May 16, 2007, NBTY, Inc.
Recalls & Warnings
June 28, 2014
Chia Seed Recall Expanded
On June 26, 2014, HEALTH MATTERS AMERICA INC.
Recalls & Warnings
June 12, 2014
Marketers of Memory Supplement Settle FTC Charges of Deceptive Claims
Martek Biosciences Corporation and i-Health Inc., marketers of BrainStrong Adult, have agreed to settle Federal Trade Commission (FTC) charges that claims the supplement could improve adult memory and prevent cognitive decline were deceptive, and not supported by sufficient clinical evidence.
Recalls & Warnings
June 05, 2014
More Chia Powders Recalled Due to Salmonella Risk
On June 4, 2014, Health Matters America Inc. issued a voluntary recall its Organic Traditions Sprouted Chia Seed Powder and Organic Traditions Sprouted Chia & Flax Seed Powder because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
May 31, 2014
Sellers of "Cancer" Supplements Warned for Drug Claims
Two sellers of graviola supplements promoted to treat cancer have been issued warning letters by the FDA. Graviola (Annona muricata), also known as soursop, is the fruit of a tree native to South America and Puerto Rico.
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
Recalls & Warnings
May 29, 2014
Recall of Sexual Enhancement Supplements Expanded
On May 28, 2014, Eugene Oregon, Inc. expanded its previous recall of certain lots of sexual enhancement supplements African Black Ant, Black Ant, and Mojo Risen. All lots of these supplements are now recalled.
Recalls & Warnings
May 21, 2014
Seller of Gingko and Milk Thistle Warned for Manufacturing Violations and Drug Claims
On April 4, 2014, the FDA issued a warning letter to Xtra Life Natural Systems, Inc.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss Supplements Warned for Manufacturing Violations and Drug Claims
On October 7, 2014, the FDA issued a warning letter to YoungYou International, Inc.
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
September 30, 2014
U.S. Marshals Seize $5 Million Worth of Kratom
On September 25, 2014, U.S. Marshals seized more than 25,000 pounds of raw kratom valued at over $5 million from Rosefield Management, Inc. in Van Nuys, California. The action was taken at the request of the FDA.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
September 09, 2014
Maker of Popular Green Coffee Bean Extract Settles FTC Charges of Unsupported Weight Loss Claims
Applied Food Sciences, Inc., maker of green coffee bean extract GCA, has agreed to pay $3.5 million to settle FTC charges that weight loss claims made about the extract were not supported by scientific evidence.
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
July 30, 2014
Chocolate and Carob Drink Mix Recalled Due To Salmonella Risk
On July 25, 2014, CaCoCo, Inc. issued a recall of CaCoCo Original and Global Warrior chocolate drink mixes because they contain organic carob powder which has the potential to be contaminated with Salmonella.
Recalls & Warnings
July 26, 2014
Marketers of Nopal Cactus Drink Settle FTC Charges of Deceptive Claims
TriVita Inc., and marketers of the company's "prickly pear" fruit drink Nopalea have agreed to pay $3.5 million in consumer refunds to settle FTC charges they made deceptive claims that the drink treats health problems ranging from skin conditions to joint pain and respiratory problems.
Recalls & Warnings
July 26, 2014
FTC Says Company Made "Outrageous" Weight Loss Claims
Marketers for the Canadian-based Freedom Center Against Obesity have agreed to pay $500,000 to settle FTC charges the company made deceptive claims about its Double Shot diet pills.
Recalls & Warnings
December 07, 2014
FDA Raises Concerns Over Generic ADHD Drugs
On November 13, 2014, the U.S.
Recalls & Warnings
November 17, 2014
Probiotic Powder Recalled
On November 14, 2014, Solgar, Inc. issued a voluntary recall of Solgar ABC Dophilus Powder (NET Wt. 1.75 oz (50 g)) because it was found to contain Rhizopus oryzae, a fungus which may cause Mucormycosis infection.
Recalls & Warnings
November 11, 2014
Maker of Children's Vitamins, Weight Loss Supplements and More Warned for Manufacturing Violations
On October 30, 2014, the FDA issued a warning letter to VitalHealth Tech, Inc., following a facility inspection which found the company's products, including Kids Mighty Vites tablets, Omni Jr.
Recalls & Warnings
February 24, 2015
Seller of Heart, Brain and Diabetes Supplements Warned for Drug Claims
On February 6, 2015, the FDA issued a warning letter to LCW, Inc.
Recalls & Warnings
January 31, 2015
Protein Powder Recalled Due to Food Poisoning Risk
On January 30, 2015, Aloha, Inc. issued a voluntary recall of all packages of Premium Protein powder blends in chocolate and vanilla because they have the potential to be contaminated with Staphylococcus enterotoxin .
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
January 21, 2015
Smoothie Blends Recalled Due to Potential Listeria Contamination
On January 18, 2015, Inventure Foods, Inc. issued a recall of RADER FARMS Fresh Start Smoothie Blend, Fresh Start Sunrise Refresh Fusion, and Fresh Start Daily Power Fusion, because they have the potential to be contaminated with Listeria monocytogenes.
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 20, 2014
Weight Loss Supplement Recalled
On December 19, 2014, Bethel Nutritional Consulting, Inc. issued a recall of one lot of weight loss supplement SLIM-K Capsules because they were found to contain sibutramine.
Recalls & Warnings
December 20, 2014
Company Recalls Second Weight Loss Supplement
On December 19, 2014, Bethel Nutritional Consulting, Inc. issued a recall of one lot of weight loss supplement B-Lipo Capsules because they were found to contain Lorcaserin.
Recalls & Warnings
March 31, 2014
Seven Sexual Enhancement Supplements Recalled Due to Undeclared Drugs
On March 27, 2014, Nova Products, Inc. issued a voluntary recall of sexual enhancement supplements African Black Ant, Black Ant, XZen Gold, ZXen Platinum, XZen 1200, XZone Gold and XZone 1200.
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
May 07, 2014
Three Sexual Enhancement Supplements Recalled
On May 5, 2014 , Eugene Oregon, Inc. issued a precautionary, voluntary recall of sexual enhancement supplements African Black Ant, Black Ant and Mojo Risen.
Recalls & Warnings
May 01, 2014
Weight Loss Supplement Recalled
On April 29, 2014, dietary supplement distributor Bacai Inc. issued a voluntary recall of one lot of weight loss supplement LiteFit USA because it was found to contain sibutramine.
Recalls & Warnings
April 29, 2014
Seller of B Vitamins, CoQ10, Amino Acids and Other Supplements Warned for Manufacturing Violations and Drug Claims
On April 1, 2014, the FDA issued a warning letter to Bio-Recovery, Inc.
Recalls & Warnings
April 24, 2014
Arthritis Supplement Containing Multiple Drugs Recalled
On April 21, 2014, Nano Well-being Health Inc., issued a voluntary recall of two lots of arthritis supplement Super Arthgold, because it was found to contain the drugs chlorzoxazone, diclofenac and indomethacin.
Recalls & Warnings
April 18, 2014
FDA Questions Ingredient in Workout Supplement
On April 4, 2014, the FDA issued a warning letter to Driven Sports Inc., following a label review which found the company's workout supplement, CRAZE, to be adulterated because it contains the new dietary ingredient Dendrobex.
Recalls & Warnings
February 19, 2014
Seller of Sexual Enhancement Supplements Warned For Manufacturing Violations
On February 11, 2014, the FDA issued a warning letter to Maximus Niterider International Group, Inc.
Recalls & Warnings
February 13, 2014
Maker of Cholesterol and Workout Supplements Warned For Manufacturing Violations, Drug Claims and Unapproved Ingredient
On January 31, 2014, the FDA issued a warning letter to Exclusive Supplements Inc.
Recalls & Warnings
January 16, 2014
Seller of Tea Supplements and Drinks Warned For Manufacturing Violations and Drug Claims
On December 31, 2013, the FDA issued a warning to Prestige Chinese Teas Company, Inc.
Recalls & Warnings
December 26, 2013
Weight Loss Supplement Recalled
On December 23, 2013, Deseo Rebajar Inc. issued a voluntary recall of one lot of Burn 7 Capsules because they were found to contain sibutramine.
Recalls & Warnings
December 19, 2013
Seller of Sexual Enhancement Supplement and Tea Drink Warned for Drug Ingredients and Drug Claims
On December 5, 2013, the FDA issued a warning letter to Green Planet, Inc. following a facility inspection which found the company's sexual enhancement supplement Night Bullet to contain the drugs sulfohydroxyhomosildenafil, thioaildenafil and aminotadalafil.
Recalls & Warnings
December 16, 2013
Weight Loss Capsules Recalled Due Allergen Risk
On December 13, 2013, Stone Independent Research, Inc. issued recall of one lot of Zanocap Scientific Weight Loss 500 mg capsules because they contain undeclared whey protein, which is derived from milk.
Recalls & Warnings
December 13, 2013
Maker of Menopause Blend and Other Supplement Ingredients Warned by FDA of Violations
On November 21, 2013, the FDA issued a warning letter to Raw Deal, Inc.
Recalls & Warnings
November 21, 2013
Weight Loss Supplement Recalled
On November 14, 2013, Deseo Rebajar Inc. issued a recall of one lot of weight loss supplement Adipotrim XT because it was found to contain undeclared fluoxetine.
Recalls & Warnings
November 20, 2013
$2 Million In Weight Loss and "Fat-Burning" Supplements Seized
On November 12, 2013, U.S. Marshals seized more than $2 million worth of supplements from Georgia-based Hi-Tech Pharmaceuticals, Inc.
Recalls & Warnings
April 09, 2014
Maker of Relaxation Drink Warned For Melatonin Ingredient
On March 27, 2014, the FDA issued a warning letter to Dewmar International BMC, Inc. following a review of the company's product packaging and labels, which found the "relaxation beverage" Lean Slow Motion ...
Recalls & Warnings
January 04, 2014
Smoking Cessation and Anti-Inflammatory Supplements Contain Unapproved Ingredient
On December 13, 2013, the FDA issued a warning letter to Star Scientific, Inc. after the agency noted that the company's smoking cessation supplement CigRx and anti-inflammatory supplement Anatabloc contain anatabine, a chemical not currently allowed in dietary supplements.
Recalls & Warnings
October 12, 2013
Metagenics Enzyme Supplement Recalled Due To Drug Risk
On October 11, 2013, Metagenics Canada issued a voluntary recall of digestive enzyme supplement Spectrazyme, due to potential contamination with the prescription antibiotic chloramphenicol.
Recalls & Warnings
October 11, 2013
Prebiotic and Digestive Enzyme Supplement Recalled Due To Drug Risk
On October 10, 2013, Adeeva Nutritionals Inc. issued a voluntary recall of digestive supplement Flora Essentials due to potential contamination with the prescription antibiotic chloramphenicol.
Recalls & Warnings
September 27, 2013
Company Warned For Distributing Weight Loss Supplement Containing DMAA, Drug Claims
On September 6, 2013, the FDA issued a warning letter to Pure Energy Products, Inc., following a facility inspection which found that the company distributes a weight loss supplement, called obestrim, which contains dimethylamylamine, or DMAA.
Recalls & Warnings
August 27, 2013
FTC Refunds Consumers of Children's Vitamins as Part of Deceptive Advertising Settlement
On August 8, 2013, the Federal Trade Commission (FTC) announced it has mailed 10,144 refund checks to consumers who purchased Disney or Marvel Hero -themed children's vitamins, including Disney Princesses, Winnie the Pooh, Finding Nemo, and Spider-Man varieties, between May 1, 2008 and September ...
Recalls & Warnings
August 23, 2013
Sexual Enhancement Supplement Containing Undeclared Drugs Recalled
On August 12, 2013, Jack Rabbit Inc. issued a voluntary recall of one lot of sexual enhancement supplement Jack Rabbit because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
August 16, 2013
Maker of Energy, Weight Loss, Sleep and Vitamin D Supplements Warned for Manufacturing Violations
On July 22, 2013, the FDA issued a warning letter to N.V.E. Pharmaceuticals, Inc.
Recalls & Warnings
July 12, 2013
Herbal Supplement Company Warned For Drug Claims, Manufacturing Violations
On June 18, 2013, the FDA issued a warning letter to Herbs of Light, Inc.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
August 06, 2013
Weight Loss Supplement Recall Expanded
On August 5, 2013, Bethel Nutritional Consulting, Inc., issued a voluntary recall of herbal weight loss supplements Quick Thin and Bethel Advance because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
April 18, 2013
Cardio, Energy and Sexual Enhancement Supplement Distributor Warned For Manufacturing Violations and Drug Claims
On December 21, 2012, the FDA issued a warning letter to ForMor Inc, dba ForMor International, following a facility inspection which found the company's Cardio Cocktail and Argenix dietary supplements to be adulterated because because they were prepared, packed, or held under conditions that do ...
Recalls & Warnings
April 11, 2013
Maker of Workout Booster Warned For Manufacturing Violations
On March 5, 2013, the FDA issued a warning letter to Primarch Manufacturing, Inc.
Recalls & Warnings
April 10, 2013
Recall: Sexual Enhancement Supplement Containing Undeclared Drug
On April 1, 2013, Consumer Concepts, Inc. issued a voluntary nationwide recall of ROCK-It MAN Male Enhancement Capsules, a dietary supplement promoted for sexual enhancement which was found to contain undeclared hydroxythiohomosildenafil, an analogue of the prescription drug sildenafil.
Recalls & Warnings
August 29, 2013
Seller of CELLFOOD Supplements Warned For Manufacturing Violations and Drug Claims
On August 1, 2013, the FDA issued a warning letter to supplement retailer Lumina Health Products, Inc.
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
June 12, 2013
Weight Loss Supplement Recalled Due To Undeclared Drugs
On June 11, 2013, Bethel Nutritional Consulting, Inc. issued a voluntary recall of one lot of Bethel 30 herbal weight loss supplement because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
June 07, 2013
Maker of Colloidal Silver and Mushroom Extract Supplements Warned For Manufacturing Violations and Drug Claims
On May 2, 2013, the FDA issued a warning letter to Earthborn Products, Inc.
Recalls & Warnings
May 29, 2013
Seller of Colloidal Silver, Noni Juice and Noni Supplements Warned For Drug Claims
On May 16, 2013, the FDA issued a warning letter to Matrix Health Products, Inc.
Recalls & Warnings
May 24, 2013
Seller of Liquid Minerals, Joint Care and Herbal Supplements Warned For Manufacturing Violations, Drug Claims
On April 8, 2013, the FDA issued a warning letter to Body Systems, Inc.
Recalls & Warnings
May 23, 2013
Maker of Liver Detox and Insulin Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to Glucorell, Inc.
Recalls & Warnings
May 17, 2013
Maker of Cardio, Arthritis, Cleanse Supplements and More Warned For Manufacturing Violations and Drug Claims
On May 8, 2013, the FDA issued a warning letter to Entrenet Nutritionals, Inc.
Recalls & Warnings
May 17, 2013
Hoodia, Sexual Enhancement Supplements and More Found To Be Adulterated, Misbranded
On March 01, 2013, the FDA issued a warning letter to Desert Rose Manufacturing, Inc.
Recalls & Warnings
February 14, 2013
Maker of Antioxidant and Anti-Aging Supplements Warned For Manufacturing Violations
On January 10, 2013, the FDA issued a warning letter to dietary supplement manufacturer Consolidated Marketing Unlimited, Inc.
Recalls & Warnings
February 12, 2013
Judge Orders Drug and Supplement Company to Cease Manufacturing and Distribution
On February 8, 2012, the FDA announced Titan Medical Enterprises Inc. has been ordered to stop manufacturing and distributing drugs and dietary supplements until the company's manufacturing operations comply with the Federal Food, Drug, and Cosmetic Act. The order was signed on December 11, 2012, U.
Recalls & Warnings
January 30, 2013
Seller of Omega-3 and Emu Oil Supplements Warned For Drug Claims, Misbranding
On November 19, 2012, the FDA issued a warning letter to Emu Products & Management, Inc. after a review of the company's website found statements that promoted the dietary supplements Multi-Omega Gel Caps, Recovery Gel Caps, ARP Gel Caps and the topical skin products Extreme Cryo Gel, F.A.C.E.
Recalls & Warnings
January 18, 2013
Recall: Iron Supplement Containing Motion Sickness Drug
On January 18, 2012, Advance Pharmaceutical Inc. announced the recall of one lot of Rugby NATURAL IRON SUPPLEMENT Ferrous Sulfate Tablets 325 mg, because the bottles were found to contain meclizine HCl 25 mg tablets instead.
Recalls & Warnings
March 22, 2013
Maker of Nutrition Shakes Warned For Manufacturing Violations
On February 28, 2013, the FDA issued a warning letter to Healthwest Minerals, Inc., dba Mt. Capra Products, Mt. Capra Whole Foods, and Mt.
Recalls & Warnings
March 21, 2013
Probiotic Recalled Due To Undeclared Soy
On March 20, 2013, New Chapter, Inc. issued a voluntary recall of one lot of its Probiotic Elderberry dietary supplement because it may contain undeclared soy.
Recalls & Warnings
March 13, 2013
Recall: Male Sexual Enhancement Supplement Containing Drugs
On March 12, 2013, Green Planet, Inc. issued a recall of one lot of the male sexual performance enhancer supplement Night Bullet because it was found to contain trace amounts of sulfohydroxyhomosildenafil and aminotadalafil, analogues of the prescription drug sildenafil.
Recalls & Warnings
March 11, 2013
Nutrition Bar Recalled Due To Undeclared Allergen
On March 7, 2013, Lifestyle Evolution Inc. issued a recall of various NuGO nutrition bars because the bars may contain undeclared milk, a potential allergen.
Recalls & Warnings
March 06, 2013
Protein Bar Recalled Due To Salmonella Risk
On March 4, 2013, Pro-Amino International Inc. issued a recall of Proti Diet High Protein Chocolate Dream Bar due potential Salmonella contamination.
Recalls & Warnings
September 07, 2012
Prebiotics Recalled For Salmonella Risk
On September 4, 2012, Eco Health, Inc. announced a recall of its florAlign Prebiotic Formula (Powder) due to the potential for the product to be contaminated with Salmonella.
Recalls & Warnings
September 21, 2012
Sexual Enhancement Supplements For Men and Women Recalled Due to Prescription Drug Risk
On August 23, 2012, Evol Nutrition Associates Inc./Red Dawn issued a voluntary recall of Mojo Nights after FDA testing revealed the male sexual enhancement supplement contains undeclared prescription drugs tadalafil and sildenafil.
Recalls & Warnings
September 18, 2012
Men’s Sexual Enhancement Supplement Containing Prescription Drug Recalled
On September 12, 2012, Body Basics Inc. issued a voluntary nationwide recall of ACTRA-Sx 500 Dietary Supplement Capsules after independent laboratory testing confirmed the supplement contains the prescription drug sildenafil citrate.
Recalls & Warnings
September 18, 2012
Maker of Noni, Nopal, Blood Sugar and Cholesterol Supplements Warned For Drug Claims, Misbranding, and Manufacturing Violations
On August 27, 2012, the FDA issued a warning letter to Naturavit, Inc. for making statements about dietary supplements Noni Imperial Hawaiian, Garlic and Parsley, Cholestol, Nopal and Diatrin that constitute drug claims.
Recalls & Warnings
August 29, 2012
Herbal Supplement Company Warned For Medical Claims
On August 2, 2012, the FDA issued a warning to HSAC Enterprises, Inc. dba Kare-N-Herbs subsequent to a facility inspection and website review in May 2012 which found statements made about Kold Kare, Energy Kare and Tranquility Kare dietary supplements to constitute drug claims.
Recalls & Warnings
August 09, 2012
Makers of Mineral Water Containing Lithium Warned
On July 20, 2012, the FDA issued a warning letter to Lithia Mineral Water, Inc. declaring Lithia mineral water product to be an approved drug following a 2011 facility inspection.
Recalls & Warnings
October 24, 2012
Warning Issued to Memory Supplement Maker For Unapproved Drug Ingredient, Drug Claims and Unreported Adverse Events
On October 16, 2012, the FDA issued a letter to dietary supplement manufacturer Quincy Bioscience Manufacturing Inc.
Recalls & Warnings
October 18, 2012
Natural Product Manufacturer Warned For GMP Violations, Misbranding, Unapproved Devices and Drug Claims
On August 28, 2012, the FDA issued a warning letter to Creation's Garden Natural Products, Inc. following an inspection of the company's manufacturing facility, which found violations of Current Good Manufacturing Practices (CGMPs) for dietary supplements.
Recalls & Warnings
November 21, 2012
Maker of Blood Pressure, Probiotic, Resveratrol and Hormonal Supplements Warned For Manufacturing Violations and Drug Claims
On November 2, 2012, the FDA issued a warning letter to Atrium, Inc. following a facility inspection which found a number of the company's dietary supplements to be adulterated or promoted as drugs.
Recalls & Warnings
February 28, 2013
Maker of Joint, Mood and B Vitamin Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On February 11, 2013, the FDA issued a warning letter to Kreativ Health, Inc.
Recalls & Warnings
December 19, 2012
Sexual Enhancement Supplements Recalled Due To Undeclared Drugs
On December 17, 2012, Performance Plus Marketing, Inc.
Recalls & Warnings
October 24, 2012
Manufacturer of Green Tea, Vitamin E, Omega-3 and Cranberry Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On October 16, 2012, the FDA issued a warning letter to dietary supplement manufacturer Advanced Nutritional Technology Inc.
Recalls & Warnings
November 02, 2012
Manufacturer of Prostate, Inflammation and Cholesterol Supplements Warned For Drug Claims
On October 15, 2012, the FDA issued a warning letter to Ortho Molecular Products, Inc. for making drug claims about the following dietary supplements: Candicid Forte, Inflamma-bLOX, Lipitrol, Mucosagen, Prostatrol Forte, Resvoxitrol, Paracid Forte, VascuPak HTN and Lithium Orotate.
Recalls & Warnings
July 27, 2012
Prescription and Street Drug Alternatives Found in Male Enhancement, Mood, and Sleep Supplements
On July 10, 2012, the FDA issued a warning letter to Evol Nutrition Associates, Inc. subsequent to a 2011 inspection which found a number of the company's products contained prescription and investigational drugs and were misbranded.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
February 20, 2013
Cold, Flu and Stress Supplement Company Warned For Adulteration, Drug Claims And More
On October 5, 2012, the FDA issued a warning letter to dietary supplement manufacturer Wholistic Herbs, Inc. following a facility inspection which found the company's products, including At-Ease, Morning Calm, Aller-Ban, Stomach Flu, Kold & Koff, Kold Sore, and L.
Recalls & Warnings
March 01, 2013
Manufacturer of Joint, Weight Loss, Muscle Supplements and More Warned for Adulteration, Misbranding and Drug Claims
On February 4, 2013, the FDA issued a warning letter to DC Nutrition, Inc.
Recalls & Warnings
May 10, 2013
Maker of Concentration, Pain, Immune, Cholesterol Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to EuroPharma Co., Inc., following a review of the company's website which found statements made about Calm Kids, CholestCaps, CuraMed 375 mg, CuraMed 750 mg, Curamin, Mental Advantage, Tri-Iodine, and Viragen to be drug claims.
Recalls & Warnings
May 03, 2013
Maker of Omega-3, Saw Palmetto, St. John’s Wort Supplements And More Warned For Manufacturing Violations and Drug Claims
On March 19, 2013, the FDA issued a warning letter to Sunset Natural Products Inc.
Recalls & Warnings
August 02, 2013
Vitamin B, Vitamin C and Multimineral Supplements Recalled Due To Anabolic Steroid Risk
On July 31, 2013, Purity First Health Products, Inc.
Recalls & Warnings
August 16, 2013
Ginkgo, Milk Thistle, Cleanse and Nopal Supplement Maker Warned For Manufacturing Violations, Drug Claims
On July 23, 2013, the FDA issued a warning letter to Natural Products Services, Inc.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
January 09, 2014
Four Companies Settle FTC Charges of Deceptive Weight Loss Claims
On January 7, 2014, the Federal Trade Commission (FTC) announced that marketers for four weight loss products have agreed to settlements over charges of deceptive weight loss claims.
Recalls & Warnings
April 16, 2014
Weight Loss Supplements Found To Contain Prescription Antidepressant and Other Drugs
On March 7, 2014, the FDA issued a warning letter to Deseo Rebajar Inc. because the company's following weight loss products were found to contain drugs:
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
September 05, 2014
Seller of "Nerve", "Cleanse" and Probiotic Supplements Warned for Drug Claims
On July 30, 2014, the FDA issued a warning letter to Plexus Worldwide, Inc., following a review of the company's website and product labels, which found statements made about Fast Relief, BioCleanse and ProBio5 to be drug claims.
Recalls & Warnings
May 10, 2007
FDA Warns of Two Supplements Containing Pharmaceutical-like Compounds
On May 10, 2007, the FDA advised consumers not to purchase or use "True Man" or "Energy Max" products promoted and sold as dietary supplements throughout the United States.
Recalls & Warnings
December 30, 2007
FDA Warns Consumers Not to Use Several "Shangai" Sexual Enhancement Supplements
On December 28, 2007, the U.S. Food and Drug Administration (FDA) advised consumers not to buy or use Super Shangai, Strong Testis, Shangai Ultra, Shangai Ultra X, Lady Shangai, and Shangai Regular, also marketed as Shangai Chaojimengnan, products.
Recalls & Warnings
February 27, 2009
$4 Million Settlement by Supplement Maker for False Claims
On February 26, 2009, the Texas Attorney General announced that an agreement was reached with Mannatech Inc. and its former CEO, Samuel L. Caster, both of which had been charged with orchestrating an unlawful marketing scheme that exaggerated their products’ health benefits.
Recalls & Warnings
July 29, 2008
Recall of Sexual Enhancement Supplements Containing Drug
On July 28, 2008 the U.S. FDA announced that Jack Distribution, LLC and G & N works, Inc. are conducting a voluntary nationwide recall of all lot numbers of the company's supplement products sold under the brand names Rize 2 The Occasion and Rose 4 Her.
Recalls & Warnings
July 28, 2008
Seizure of Xiadafil VIP Tablets After Company Refuses Recall
On July 24, 2008, the FDA announced that U.S. Marshals seized nearly $74,000 worth of Xiadafil VIP tablets, Lots 6K029 and 6K209-SEI, distributed by SEI Pharmaceuticals, Inc. of Miami, Fla.
Recalls & Warnings
January 16, 2010
Recall of Body Building Supplements Containing Steroids
On January 15, 2010, the Food and Drug Administration (FDA) posted a voluntary recall notice from MuscleMaster.com, Inc. regarding all lots and expiration dates of seventeen dietary supplements sold in 2009 from June 1 and Novemember 17.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
June 15, 2009
Sexual Enhancement Supplement Recalled -- Second Time Found with Drug-like Compound
On June 15, 2009, the U.S. FDA announced that, per its order, Hi-Tech Pharmaceuticals, Inc. is conducting a nationwide voluntary recall of the company's product sold under the name Stamina-Rx.
Recalls & Warnings
March 11, 2003
Rexall Agrees to Pay up to $12 million to Users of Misleading Cellulite Supplement
On March 11, 2003, the Federal Trade Commission (FTC) reported that Rexall Sundown, Inc. (Rexall) will pay up to $12 million to resolve FTC charges regarding its marketing of the dietary supplement, "Cellasene," a purported cellulite treatment product.
Recalls & Warnings
May 28, 2003
Warning and Recall for "Herbal" Sexual Enhancement Supplement in Canada Illegally Containing Pharmaceutical Compound
On May 27, 2003 - Health Canada warned consumers not to use Hua Fo VIGOR-MAX tablets, a Chinese herbal product that contains tadalafil.
Recalls & Warnings
August 16, 2006
Marketers of Diabetes Supplement Banned From Claiming Products Treat or Cure Diseases
On August 10, 2006, the Federal Trade Commission (FTC) announced that an operation selling Chinese herbal supplements is banned from claiming its products treat or cure diseases, to settle FTC charges it violated a previous court order.
Recalls & Warnings
December 22, 2005
Health Canada Warns Consumers Not to Take Chaparral
On December 21, 2005, Health Canada (Canada's health ministry) warned consumers not to ingest the herb chaparral in the form of loose leaves, teas, capsules or bulk herbal products because of the risk of liver and kidney problems.
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
June 17, 2004
FTC Challenges Deceptive Weight Loss Claims for Supplement Targeted at Hispanics
On June 17, 2004, the Federal Trade Commission (FTC) announced a new law enforcement action challenging false advertising for the 1-2-3 Diet Kit, a purported weight loss product.
Recalls & Warnings
January 18, 2005
Sellers of “Fat Trapper Plus” and “Exercise in a Bottle” Banned from Advertising Weight-Loss Products
On January 18, 2005, the Federal Trade Commission (FTC)announced that Enforma Natural Products, Inc.
Recalls & Warnings
January 05, 2005
Marketers of Brain-Rejuvinating Supplement Settle FTC Charges of False Claims
On January 5, 2005, the U.S. Federal Trade Commission (FTC) announced that a company that heavily advertised an herbal dietary supplement called "Sagee" to the Chinese-language and Vietnamese-language communities had settled FTC charges of making false and unsubstantiated claims for the product.
Recalls & Warnings
October 10, 2003
Government Stops Sale of Supplements Claiming to Treat Obesity and Impotence
On October 8, 2003, The Food and Drug Administration (FDA) announced that on September 22, 2003, U.S. District Court Judge Robert J. Vining, Jr.
Recalls & Warnings
July 30, 2003
Maker of Weight-Loss and Cellulite Treatment Ordered to Stop False Claims and Pay Consumer Redress
On July 30, 2003 the Federal Trade Commission (FTC) reported that a Canadian-based company operating in the United States under the name “Bio Lab,” and its president, Jean-Francois Brochu, have agreed to settle FTC charges that they deceived consumers through false and unsubstantiated advertising ...
Recalls & Warnings
August 07, 2015
Male Enhancement and Weight Loss Supplement Containing Drugs Recalled
On August 6, 2015 Blue Square Market Inc. issued a recall of the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.





