Product Reviews and Information for Stroke
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Which supplements reduce the risk of stroke? Which increase the risk of stroke?
Find out which supplements (may help reduce the risk of stroke/might increase the risk of stroke.)

Product Review
Choline and Lecithin Supplements Review (Including Phosphatidylcholine, CDP-Choline, and Alpha-GPC)
Choose the Best Choline Supplement. Find Out How Much Choline Popular Supplements Really Provide.

Product Review
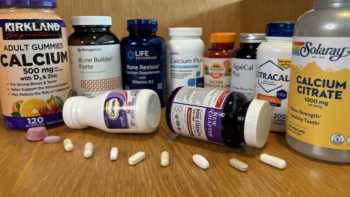
Calcium and Bone Health Supplements Review (Including Vitamins D & K, Magnesium and Boron)
See Which Bone Health Supplements Are Top Picks and Which Fail
Product Review
Green Tea Review: Tea Bags, Loose Leaf Tea, Matcha Powders, and Supplements
Some green teas provide barely any green tea polyphenols, while some others are high strength. See the Test Results and Our Top Picks for Green Tea.

CL Answer
What is lumbrokinase, does it have heart benefits, is it safe, and what should you look for, or avoid, in a lumbrokinase supplement?
Lumbrokinase is marketed as promoting healthy circulation and supporting heart health. Find out how and if lumbrokinase supplements actually work, possible safety concerns, and what to consider when choosing a lumbrokinase supplement.

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
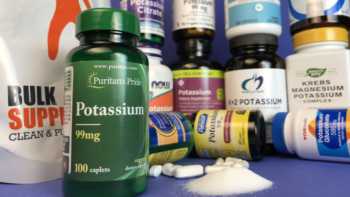
Potassium Supplements Review
Be Careful with Potassium Supplements! Problems Found. Tests and Reviews of Potassium Supplements & CL's Top Picks.
CL Answer
Do "lite" salts and salt substitutes containing potassium help lower blood pressure and reduce heart risks? Are they safe?
Find out if replacing regular salt with "lite" salt or salt substitutes, such as Morton Lite Salt, Morton Salt Substitute, NoSalt Original Sodium Free Salt Alternative, and MySalt, helps to reduce blood pressure, or decrease the risk of heart attack and stroke. Plus, learn about the safety and side effects associated with salt substitutes, drug interactions, and more.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
Iron Supplements Review (Iron Pills, Liquids and Chews)
See Which Iron Supplements Are CL's Top Picks for Different Needs

CL Answer
Does I3C (from broccoli) or DIM (a related chemical) reduce the risk of cancer?
Find out if the compound indole-3-carbinol (I3C), from broccoli, and its metabolite diindolylmethane (DIM) can reduce the risk of cancer. ConsumerLab.com's answer explains.

CL Answer
What is Noopept? Can it really improve memory and cognition, and is it safe?
Learn more about Noopept, including results from clinical studies on cognition, safety, and dosage.
CL Answer
What is hesperidin, what is it used for and is it safe?
Learn about hesperidin, including whether its beneficial for heart health, problems with circulation, stroke, cognitive function, depression, cancer and other conditions, and find out if it is safe.

Clinical Update
2/18/2022
Do Vitamin D & Whey Protein Help After a Stroke?
Can taking vitamin D and whey protein after a stroke help improve muscle strength and physical function? Find out what a recent study suggests in the Muscle, balance and falls section of our Vitamin D Supplements Review.
Also see our article about supplements and stroke risk.
Clinical Update
2/06/2025
Benefit of Salt Substitute After Stroke
Among people who have had a stroke, switching from regular table salt to a potassium-enriched salt substitute may greatly reduce the risk of another stroke and death. Get the details and our list of good "lite" salts and salt substitutes in our Potassium Supplements Review.
Clinical Update
12/09/2022
Supplement Linked with Stroke
DIM (diindolylmethane), a supplement promoted for treating cancer, has been linked with reports of clots and stroke. Get the details in our updated CL Answer, "Does I3C (from broccoli) or DIM (a related chemical) reduce the risk of cancer?”
Clinical Update
11/18/2012
Stroke and Fish Oil
An analysis of 38 studies concluded that eating fish regularly is associated with fewer strokes, but fish oil supplements do not seem to help. Interestingly, the type of fish and how it is prepared seem to make all the difference. For details, including what fish oil supplements can or cannot do, see the updated Fish Oil & Marine Oil (Omega-3) Supplements Review. More >>
Clinical Update
6/24/2012
Fish Oil for Strokes and Heart Attacks?
A recent study with fish oil supplements found no reduction in strokes and heart attacks among people with diabetes and poor glucose control. Does this mean fish oil doesn't help with cardiovascular disease? Not necessarily. See the updated Fish Oil (Omega-3) Supplements Review. More >>
Clinical Update
2/26/2012
Less Stroke With More Magnesium
Slightly higher intake of magnesium from the diet was associated with an 8% lower risk of stroke, based on an analysis of several studies. This doesn't necessarily mean you should get more magnesium from supplements, though. For details, see the updated information in the Magnesium Supplement Review. More >>
Clinical Update
11/20/2011
No Benefit of Niacin with Statins
According to a recently published study, high dose niacin added to statin drug treatment does not further reduce the risk of heart attacks and stroke despite improvements in levels of HDL cholesterol and triglycerides. In fact, there was a small increase in strokes among those taking niacin. However, a role remains for high dose niacin in lipid treatment. For details, see the update to the B Vitamin Supplements Review.
Clinical Update
9/21/2013
B Vitamin Supplements for Stroke?
A review of 14 clinical studies suggests that B vitamins may help prevent stroke. However, a deep reading of the report shows the benefit to be slight, at best. For details about the study, other uses of B vitamins (including B6, B-12, folic acid, niacin and complexes), and tests of these supplements, see the B Vitamin Supplements Review>>
Clinical Update
3/15/2015
Folic Acid Reduces Risk of Stroke
A large study in people taking blood pressure-lowering medication found that the addition of a daily folic acid (vitamin B-9 or folate) supplement significantly reduced the chance of having a stroke. This benefit is most likely to occur among people whose blood levels of folate are low. More details, including dosage and ConsumerLab.com's test-based quality ratings of folic acid supplements, are found in the B Vitamin Supplements Review >>
Clinical Update
5/24/2017
Stroke Risk with Calcium
A new study shows that high-dose calcium supplements may double the risk of stroke in men and women. The risk of heart attack is also increased, as shown in other studies. However, there are ways to take calcium without this risk and still get bone health benefits. For details, see the "Concerns and Cautions" section of the Calcium Supplements Review >>
Clinical Update
10/29/2018
Iron and Stroke
Having too much iron in the body is associated with an increased risk of a variety of conditions and, according to a recent study, stroke is among these. For details, see the Concerns and Cautions section of the Iron Supplements Review. (Also see our Top Picks for iron when you need to supplement.)
Clinical Update
11/12/2019
Green Tea & Stroke
Studies have found green tea consumption to be associated with a decreased risk of stroke. However, a recent study found this to be the case for only for one gender. For details, see the What It Does section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
9/04/2018
Fish Oil & Diabetes
In a major study, fish oil was given to people with type 1 or type 2 diabetes for several years to see if it would reduce serious cardiovascular events, like heart attacks and stroke. Find out what happened in the Heart Attack and Stroke section of the Fish Oil Supplements Review. (Also see our Top Picks among fish oil supplements.)
Clinical Update
3/06/2021
Melatonin After Stroke?
Melatonin supplementation may reduce the risk of developing delirium after a stroke, according to a new study. Get the details in the What It Does section of our Melatonin Supplements Review. Also see our Top Picks among melatonin supplements.
Clinical Update
5/22/2021
Does Iron Affect Stroke Risk?
There are downsides to taking too much iron, but a recent analysis suggests that getting a basic amount of iron may reduce stroke risk for women. Get the details in the Concerns and Cautions section of our Iron Supplements Review.
Clinical Update
9/13/2024
Alpha-GPC and Stroke Risk?
Does alpha-GPC, a source of choline, increase stroke risk or blood levels of TMAO (which has been linked with adverse cardiovascular effects)? Find out in our article about choline and TMAO.
CL Answer
Does glucosamine raise "bad" LDL cholesterol?
Learn more about the link between glucosamine and cholesterol, including evidence from clinical studies.

CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

CL Answer
Is it better to use a powdered product that you mix with liquid than to take a pill? I was told that the vitamins will be more completely absorbed. Is that true?
Learn more about taking supplements & multivitamins in powder or tablet form, including tips on how to get the most from your supplement.

CL Answer
Is it better to take fish oil, flaxseed oil -- or both?
Fish oil and flaxseed oil -- how they are different, what they are used for, if and how they can help with arthritis, depression, lowering cholesterol & more.

CL Answer
I enjoy oat milk in my coffee, but how healthy is it for me?
Is oat milk healthy? Information about oat milk contents, how much sugar and calcium is in oat milk, and if it is a healthy alternative to cow milk.

Clinical Update
2/03/2018
Disheartening Fish Oil News
Another report was published this week showing no cardiovascular benefit from fish oil supplementation. You can get the details in the Heart Attack and Stroke section of the Fish Oil Supplements Review. Fish oil may provide cardiovascular benefit in limited situations, as explained in the Review.
Clinical Update
2/04/2017
Many Benefits of Magnesium
An analysis of studies involving more than one million people found that rates of stroke, heart failure, type 2 diabetes, and death were lower when intakes of magnesium were higher. For details see the "What It Does" section of the Magnesium Supplements Review (which includes our tests of products) >>
Clinical Update
3/19/2019
Prescription Fish Oil Lowers Heart Attack Risk
A prescription fish oil was found to lower the risk of cardiovascular events such as heart attack and stroke by 30% in people at high risk. For details, see the Prescription Fish Oil vs. Supplements section of the Fish Oil Supplements Review. Also see our Top Picks for fish oil supplements.
Clinical Update
7/02/2019
Licorice Danger
A woman suffered a stroke after consuming a supplement for indigestion that contained a large dose of licorice. Get more details in our answer about licorice tea.
Clinical Update
8/25/2020
Calcium & Artery Calcification
Is there an increased risk of coronary artery calcification with the use of calcium supplements? Find out what a recent analysis showed in the Heart Attack and Stroke Risk section of the Calcium Supplements Review. Plus, learn how much calcium one needs per day and how much, if any, can come from supplements. Also see our Top Picks for calcium.
Clinical Update
1/23/2018
A Reason to Take Fish Oil With Statins?
It's well known that high-dose fish oil can lower elevated triglycerides. But can it help people already on a statin drug? A recent clinical trial answered this question. Get the details, including the dose of fish oil used, in the "Heart Attack and Stroke section of the Fish Oil Omega-3 Fatty Acid Supplements Review. (Also see our Top Picks among products.)
Clinical Update
7/10/2018
Multivitamins & Cardiovascular Disease
Does taking a multivitamin reduce the risk of heart disease and stroke? See what a recent analysis concluded in the Background section of the Multivitamin Review. (Also see our Top Picks among multivitamins.)
Clinical Update
7/21/2018
Fish Oil for Heart Health?
Are fish oil supplements good for the heart? See what a review of over 70 clinical trials concluded in the Heart Attack and Stroke section of the Fish Oil and Omega-3 Supplements Review.
Also, learn what fish oil can or cannot do for other health conditions, such as arthritis, in the What It Does section of the review. (See our Top Picks among fish and marine oils.)
Clinical Update
3/14/2017
When Does Fish Oil Help the Heart?
There are new recommendations on the use of fish oil supplements with regard to preventing heart attack, stroke, atrial fibrillation, and heart failure. More about these recommendations and other uses of fish oil are found in the "What It Does" section of the Fish Oil Supplements Review (which includes our tests of products) >>
Clinical Update
5/30/2017
Heart Risk Lower with Chocolate
Eating chocolate is associated with a reduced risk of developing atrial fibrillation, according to a large, long-term study. Atrial fibrillation is associated with higher risk of stroke, heart failure and other serious problems. Details about this and other benefits of chocolate are found in the "What It Does" section of the Chocolate and Cocoa Powders Review, which includes our findings for the flavanol content and contamination levels of popular products.
Clinical Update
5/22/2018
Eat Fish for Heart Health
The American Heart Association recently affirmed that eating fish may help to reduce the risk of cardiac death, coronary heart disease, and ischemic stroke. However, the same cannot be said for fish oil supplements. Get the details in the What It Does section of the Fish Oil Supplements Review. Also, stay tuned: ConsumerLab is currently testing popular brands of canned tuna and salmon to assess their purity and levels of omega-3 fatty acids.
Clinical Update
4/22/2012
Heart Benefits of Fish Oil Supplements in Doubt
A review of the latest studies finds no conclusive evidence that fish oil supplements help people hoping to prevent another heart attack or stroke. It also remains unclear if fish oil provides cardiovascular benefits for healthy individuals. Find out what people concerned about heart disease should do, the other potential benefits of fish oil supplements, and our tests of supplements on the market in the updated Fish Oil Supplements Review. More >>
Clinical Update
5/19/2013
Fish Oil No Help for Heart Disease?
A new study found that taking a fish oil supplement did not reduce the risk of heart attack or stroke in people with multiple risk factors for heart disease. But this doesn't tell the whole story. Get the bottom line on fish oil for heart disease and test results for over 70 fish oil supplements, in the updated Fish Oil and Omega-3 Fatty Acid Supplements Review >>
Clinical Update
11/11/2012
Multivitamin and the Heart
Does taking a daily multivitamin reduce the risk of heart attack and stroke? No, according to a recently published, major U.S. study. Those who took the popular multi, however, were more likely to report a particular side effect than those who took placebo. For details, see the update in the Multivitamin Supplements Review. More >>
Clinical Update
9/16/2012
Fish Oil Benefit Questioned
There are many potential benefits from supplementing with the omega-3 fatty acids found in fish oil, but the most widely-touted benefits -- reducing the risks of heart disease and stroke -- are now in doubt. Read about the latest studies in the update to the Fish Oil (EPA & DHA) Supplements Review, which includes our product tests and quality ratings. More >>
Clinical Update
1/09/2024
High-Dose Fish Oil for Heart Attack Recovery?
During recovery from heart attack, does high-dose fish oil prevent another heart attack, heart failure, or death? Find out what a recent study showed in the Heart Disease and Stroke section of our Fish Oil Supplements Review. Also see our Top Picks for fish oil.
Clinical Update
2/21/2024
Big Benefits of Salt Substitute
A study among older people with high blood pressure showed that use of a salt substitute led to a dramatic reduction in heart attack and stroke. Get the details in the Salt Substitutes section of our Potassium Supplements Review, which includes information about popular salt substitutes on the market.
Clinical Update
3/29/2024
Microplastics Cardiovascular Concern
Microplastic particles were recently found in arterial plaque in many people, and this was associated with much higher risk of stroke, heart attack, and death. Get the details in the Microplastics section of our Water Filter Pitchers Review. Also see our Top Pick water filter pitcher for removing microplastics.
Clinical Update
3/03/2023
Heart Risk from Erythritol Sweetener?
High intake of erythritol was linked to an increased risk of heart attack and stroke in a recent analysis. Should you be concerned? Find out in the Erythritol section of our CL Answer about sweeteners.
Clinical Update
2/23/2022
Coffee and Heart Health
Drinking moderate amounts of one type of coffee, but not another, was linked to a reduced risk of heart attack and stroke in a recent analysis. Get the details in our updated article about Coffee and Health.
Clinical Update
3/01/2022
Heart Risk With Fizzy Pills and Powders
Be aware that supplements that "fizz" in water may be exposing you to potentially dangerous amounts of sodium. This was associated with higher risk of heart attack, stroke, or heart failure in a recent study.
Clinical Update
3/15/2022
Fish Oil and Heart Failure Risk?
Can supplementing with fish oil reduce cardiovascular risk factors in people at high risk for heart failure? Find out what a recent study showed in the Heart Attack and Stroke section of our Fish and Marine Oil Supplements Review. Also see our Top Picks among fish oil supplements.
Clinical Update
4/05/2022
Fish Oil for Heart Failure?
Can fish oil reduce the risk of being hospitalized due to heart failure? See what a major study found in the Heart Disease and Stroke section of our Fish Oil Supplements Review. Also, see our Top Picks for fish oil.
Clinical Update
8/15/2024
Erythritol Risk in Dark Chocolate?
Some dark chocolate contains erythritol, which has been linked to higher risk of stroke. Find out if the amount of erythritol in Lily’s dark chocolate poses a risk in our Dark Chocolates and Cocoas Review.
Clinical Update
5/10/2024
Eggs and Risk of Death
A recent study found that eating more than a certain number of eggs (a source of choline) daily carried an increased risk of death among people with a history of heart disease or stroke. Get the details in the Concerns and Cautions section of our Choline Supplements Review.
Clinical Update
6/11/2024
Xylitol & Heart Risk
The sweetener xylitol was recently linked with an increased risk of heart attacks, stroke, and death, although this does not prove cause-and-effect. Get the details in our article about sugar substitutes, which includes information about xylitol and 15 other low-calorie sweeteners.
Clinical Update
6/14/2024
Is Xylitol in Toothpaste Unsafe?
Use of Xylitol was recently linked with increased risk of heart attacks, stroke, and death, but does xylitol in toothpaste or salivary stimulants for dry mouth pose a concern? Find out in our article about toothpastes and other dental products, which inlcudes our Top Picks.
Also, find out if intake of regular sugar has been linked with heart-related concerns.
Clinical Update
3/31/2025
Calcium and Heart Disease
Can a person with coronary artery disease take a calcium supplement without increasing cardiovascular risk and, if so, what dose might be safe? Find out in the Heart attack and stroke risk section of our Calcium Supplements Review. Also see our Top Picks among calcium supplements.
Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

Product Review
L-Citrulline Supplements Review
Learn How Citrulline Products Differ and Our Top Picks

CL Answer
Does Prevagen really improve memory?
Learn more about Prevagen, including clinical studies on Prevagen's impact on memory & cognition, safety, pricing, and an FDA warning letter. ConsumerLab explores the efficacy of Prevagen.

Clinical Update
6/22/2011
Does Taking Vitamin D Reduce the Risk of Heart Disease?
Earlier research has suggested an association between higher vitamin D levels in the blood and reduced risk of heart attack. But blood levels are affected by both sun exposure as well as intake of vitamin D. A new study shows that men with higher vitamin D intakes from foods and supplements have a 14% reduction in their risk of cardiovascular disease and stroke. However, the study found no reduction among women. The amount of vitamin D intake shown to be beneficial and possible reasons why a benefit was not found in women are provided in the updated "What It Does" section of our Product Review of Vitamin D Supplements. More >>
Clinical Update
5/31/2011
Niacin Concern Stops Cardiovascular Study
A clinical trial using high dose niacin (vitamin B3) was recently stopped by the National Institutes of Health due to lack of benefit and a small increase in strokes. Patients in the study had cardiovascular disease and were already taking a statin drug to keep LDL ("bad") cholesterol levels low. The addition of high dose niacin was intended to increase HDL ("good") cholesterol, reduce elevated triglycerides, and, most importantly, reduce the risk of heart attacks and stoke. For more information, including the amount and type of niacin used in the study and additional information about niacin and niacin supplements, see the B Vitamin Supplements Review. More >>
CL Answer
Risks of Too Many Vitamins & Supplements
Taking too many vitamin supplements -- or taking too much of a particular supplement -- can have short and long-term adverse effects.

Product Review
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
Product Review
Vitamin D Supplements Review (Including Calcium, Magnesium, Vitamin K, and Boron)
Find the Best Vitamin D Supplement and Avoid Problems

CL Answer
What’s the Best Way to Get High-Quality Protein from Food?
Find out the best way to get high-quality protein from animal or plant-based food instead of supplements.

CL Answer
If I already take red yeast rice, would adding high-dose niacin help further reduce my cholesterol levels, and would this be safe?
Learn more about the effect of combining red yeast, statins and high-dose niacin on cholesterol and safety.

Product Review
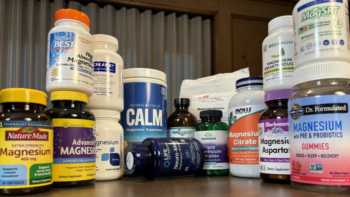
Magnesium Supplements Review (Including Calcium, Vitamins D & K, and Boron)
Find Out What Magnesium Does, Who Needs It, and Our Top Picks Among Supplements
CL Answer
What is desiccated beef liver? What is it used for, and is it safe?
Description of desiccated beef liver, including what it is used for, its nutrient content, and safety concerns.

CL Answer
Can taking too much vitamin B-6 be dangerous?
Find out if multivitamins contain excessive amounts of B-6, how much B-6 is too much, and symptoms of toxicity and overdose.

CL Answer
Does OsteoMD improve calcium absorption and increase bone density?
ConsumerLab reviews the evidence for OsteoMD by 1MD, a bone health supplement promoted to slow bone loss and reduce the risk of osteoporosis.

CL Answer
Which supplements may benefit people with essential tremor, and which should be avoided?
Learn about supplements used for treating essential tremor, including caprylic acid (also called octanoic acid), thiamin (vitamin B1), cannabidiol (CBD), GABA (gamma-aminobutyric acid), and branched-chain amino acids. Find out which work and which don't. Also find out which supplements and food may make essential tremors worse.

CL Answer
Is there a danger in taking lecithin or phosphatidylcholine? I heard that they may increase the risk of heart attacks.
Lecithin, phosphatidylcholine and choline - Learn more about the potential link between them and cardiovascular disease.

CL Answer
My HDL (good) cholesterol level is low. Should I take niacin to raise my HDLs?
Learn more about niacin and find out if it can raise HDL ("good") cholesterol levels.

CL Answer
Is drinking coffee good or bad for heart health?
Learn more about coffee and caffeine, its safety, if it's good for you, how much coffee is too much and its impact on your heart.

Product Review
Tuna, Salmon, Sardines & Herring Review (Canned and Packaged)
Find Products With the Least Mercury and Most Omega-3s

Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

Recalls & Warnings
April 25, 2024
Drug Found in Umary Supplement
On April 18, 2024, CTV News in Canada reported tests suggesting that Umary Hyaluronic Acid Dietary Supplement may be adulterated with the prescription anti-inflammatory drug diclofenac, which may explain side effects that have been reported with its use.
CL Answer
Is it better to get vitamins from foods or supplements, and are natural vitamins better than synthetic vitamins?
Learn more about the benefits of getting vitamins from food versus supplements, natural versus synthetic vitamins, and more.

CL Answer
What' the Best Way to Take Vitamin B-12? Dosage and Absorption Tips
Discover the optimal dosage and ways to take vitamin B-12, an essential nutrient, for better absorption and effectiveness and learn about possible side effects.
Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

CL Answer
Do vitamin patches, such as for B12 or multivitamins, really work? How about those from PatchMD?
Do vitamin B12 Patches like PatchMD really work? Can you absorb vitamin B12 through patches?
CL Answer
Which supplements help to improve energy and decrease fatigue?
Energy supplements and vitamins - Some can help increase energy and reduce fatigue, including CoQ10, curcumin/turmeric, cocoa, and B vitamins.

CL Answer
What are the health benefits of serrapeptase?
Serrapeptase is a proteolytic enzyme. Learn more about its health effects, including whether evidence supports its use for pain, inflammation, and heart health.

CL Answer
Which supplements or foods can help lower cholesterol and keep my heart healthy? Are there any to avoid?
Information about heart health supplements & vitamins that help with cholesterol, including sterol esters, CoQ10, and vitamin D, and which may be bad for the heart, like calcium.

Recalls & Warnings
May 01, 2024
FDA Warns Consumers Not to Use Ossos-Sans Supplement for Joints
On April 17, 2024, the FDA warned consumers not to purchase or use Ossos-Sans, a supplement promoted to treat osteoarthritis, osteoporosis, and other conditions, because it contains undeclared drugs, including diclofenac and methocarbamol.
Recalls & Warnings
April 25, 2024
Yogi Tea Recalled Due to Pesticides
On April 19, 2024, Yogi Tea issued a recall of over 54,800 packs of Yogi Echinacea Immune Support tea after a sample of echinacea tested above acceptable limits for multiple pesticides. No illnesses have been reported to date.
Recalls & Warnings
April 23, 2025
FDA Warns Consumers Not to Use ADVANCED KING Joint Supplement
On February 10, 2025, the FDA warned consumers not to purchase or use the joint pain supplement ADVANCED KING (Naturista Store LLC), because FDA analysis confirmed the product contains dexamethasone, diclofenac, and methocarbamol.
Recalls & Warnings
June 26, 2024
FDA Warns Consumers Not to Use Infla-650 Due to Undeclared Drugs
On June 20, 2024, the FDA warned consumers not to purchase or use Infla-650 after FDA laboratory analysis found them to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
July 16, 2024
Infla-650 Herbal Dietary Supplement Recalled
On July 16, 2024, Guru Inc. issued a recall of one lot of Infla-650 Herbal Dietary Capsules because they were found to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
October 08, 2024
AK Forte Joint Pain Supplement Recalled
On October 8, 2024, C&A Naturistics voluntarily recalled all lots of AK Forte, 400 mg tablets because FDA laboratory analysis found products to contain undeclared diclofenac, dexamethasone, and methocarbamol.
Recalls & Warnings
December 19, 2024
Force Forever Joint Pain Supplement Recalled
On December 12, 2024, GNMart INC recalled all lots of Force Forever, a supplement promoted for joint pain, because FDA tests found it to contain undeclared diclofenac and dexamethasone.
Recalls & Warnings
February 03, 2025
Four Male Enhancement and Energy Supplements Found to Contain Drugs
On January 16, 2025, the FDA issued a Warning Letter to Mihon Corp. d/b/a VitalityVita and Boulla, LLC after FDA analysis found four of the company’s supplements, VitalityXtra, PeakMax, ZapMax, and ZoomMax, to contain undeclared sildenafil.
Product Review
Ginkgo (Ginkgo Biloba) Supplements Review
Choose the Best Ginkgo Biloba Supplement. Finding Real Ginkgo Isn't Easy — 60% Fail ConsumerLab's Tests of Quality.

Product Review
Plant-Based Milks Review (Almond, Cashew, Coconut, Flax, Hemp, Macadamia, Oat, Pea, and Soy)
Find the Best Non-Dairy Milk Alternative. ConsumerLab Tests Reveal What's Really In Plant-Based Milks.

CL Answer
Can taking too much vitamin B-12 be dangerous? The label on my B-complex states it contains 50,000% of the Daily Value!
Find out the daily value for vitamin B-12 and the potential side effects of taking too much B-12. ConsumerLab explains why taking too much vitamin B-12 can be harmful.
CL Answer
My doctor told me to stop taking supplements because my kidney function was low. After stopping, my kidney function returned to normal. Can taking a lot of supplements really damage the kidneys?
Find out which supplements may damage your kidneys. ConsumerLab explores possible kidney side effects of supplements including chromium, creatine, and vitamin C.

CL Answer
How do I find the best quality olive oil and what are the health benefits?
Health Benefits of olive oil, including heart benefits, anti-inflammatory effects. Plus, how to choose a quality olive oil and cook with olive oil

CL Answer
How much calcium do you need each day, what forms are best, and how much is too much?
Learn about the recommended daily intake of calcium from food & supplements and why taking too much can be harmful. Also, learn about the forms of calcium that are available and which are best.

Recalls & Warnings
December 14, 2023
Pain Relief Tea Recalled, Found to Contain Prescription Drugs
On December 13, 2023, WS Global issued a recall of all lots of the company’s Himalayan Pain Relief Tea after products were found to contain diclofenac and dexamethasone, prescription drugs that are not listed on the product’s label.
Recalls & Warnings
September 25, 2023
FDA Warns Consumers Not to Use Tapee Tea
On August 31, 2023, the FDA warned consumers not to purchase or use Tapee Tea products after FDA laboratory analysis found them to contain dexamethasone and piroxicam, drugs that are not listed on the product's label.
Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
September 05, 2024
Don’t Buy or Use Umary and Amazy Supplements, Warns FDA
On September 5, 2024, the FDA warned consumers not to purchase or use Umary Hyaluronic Acid or Amazy Hyaluronic Acid supplements because testing by the agency found them to contain diclofenac and omeprazole.
Recalls & Warnings
November 07, 2024
Sexual Enhancement Supplements Sold on Amazon Recalled
On November 4, 2024, four sexual enhancement supplements sold on Amazon and other websites, ZoomMax and ZapMax (Boulla LLC) and VitalityXtra and PeakMax (VitalityVita LLC) were recalled because they were found to contain undeclared prescription drugs, ...
Recalls & Warnings
November 21, 2024
Umary Hyaluronic Acid Sold on Amazon Recalled
On November 20, 2024, MXBBB issued a recall of one lot of Umary Hyaluronic Acid or Amazy Hyaluronic Acid supplements, which were sold on Amazon.
News Release
October 17, 2024
Not All B Vitamin Supplements Contain What They Claim, According to ConsumerLab Tests
White Plains, NY, October 17, 2024 — Recent ConsumerLab tests revealed that 19% of popular B vitamin complexes and individual B vitamin supplements selected for testing contained far less, or far more of one or more B vitamins than claimed on the label.
CL Answer
What is Perque Bone Guard Forté? It is safe and effective for bone health?
Find out about the ingredients in Perque Bone Guard Forté, including which ingredients may be beneficial for osteoporosis and bone health and which may be a safety concern.

CL Answer
Does silica have health benefits? What is BioSil, and can it really increase collagen production and strengthen hair, skin, nails and bones?
Information about the health effects of silica (silicon dioxide) and orthosilicic acid (an ingredient in BioSil), including whether these forms of silicon can really increase collagen production, thicken hair, strengthen nails & bones.
CL Answer
A tea called Throat Coat made me lethargic and I was found to have low potassium. Is this a known problem? I was drinking 5 cups a day.
Throat Coat tea information, including licorice root content, potassium loss, and symptoms such as lethargy, increased blood pressure and fluid retention. ConsumerLab.com's answer explains.

CL Answer
What is TUDCA (tauroursodeoxycholic acid)? Does it have any proven health benefits, and is it safe?
TUDCA information, including its potential health benefits, safety, possible interactions, and cost.
CL Answer
Does Qualia Mind boost memory and mental function and is it worth cost?
Learn about the ingredients in Qualia Mind, whether or not we think the formula works and is safe, and if we think it's worth the cost.

Recalls & Warnings
April 27, 2022
Prescription Drugs Found in Arthritis, Muscle Pain, and Osteoporosis Supplements
On April 20th, 2022, the FDA warned consumers not to use the following ”Artri“ or ”Ortiga” products promoted to treat arthritis, muscle pain, osteoporosis and bone cancer because they have been found to contain undeclared drugs that can have serious adverse ...
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
February 13, 2023
FDA Finds Prescription Drug in “100% Natural” Weight Loss Supplement
On February 8, 2023, the FDA warned consumers not to buy or use Alfia 100% Natural Weight Loss Capsules after FDA laboratory analysis confirmed the presence of sibutramine.
Recalls & Warnings
June 16, 2022
Omega 3 Supplements Recalled Due to Undeclared Drug Ingredients
On May 28, 2022, Latin Foods Market issued a voluntary recall of one lot of Artri King Reforzado con Ortiga y Omega 3 tablets after FDA laboratory analysis found the presence of undeclared diclofenac and dexamethasone.
Recalls & Warnings
July 14, 2022
FDA Warns Company Selling Supplements with Dangerous Steroid-like Substances
On July 6, 2022, the FDA issued a warning letter to Elite Supplement Center LLC and Elite Supplement Training Facility LLC for selling the following products labeled as containing steroid-like substances known as selective androgen receptor modulators (SARMs), which are not permitted in dietary ...
Recalls & Warnings
June 22, 2023
FDA Warns Warrior Labz for Selling Products with Steroid-Like Substances and Prescription Drugs
On June 12, 2023, the FDA issued a warning letter to Warrior Labz SARMS following inspection of the company’s website and social media, which found that the company sold the following products labeled as containing steroid-like substances known as selective androgen receptor modulators ...
News Release
July 31, 2024
Best Calcium & Bone Health Supplements According to ConsumerLab Tests
White Plains, NY, July 31, 2024 — Calcium is essential to maintaining bone health and plays critical roles in nerve transmission, muscle contraction, and the cardiovascular system.
News Release
June 14, 2022
Best B Complexes, B12, and Other B Vitamin Supplements Revealed by ConsumerLab Tests: Beware of High Doses
White Plains, New York, June 14, 2022 — Recent ConsumerLab tests of over 30 popular vitamin B complexes and supplements revealed that four products contained either much less, or much more, of at least one B vitamin listed on the label.
News Release
May 16, 2022
Best Calcium and Bone Health Supplements Revealed by ConsumerLab Tests
White Plains, New York, May 16, 2022 — Calcium is essential for maintaining bones and plays critical roles in nerve transmission, muscle contraction, and the cardiovascular system.
News Release
May 26, 2017
ConsumerLab.com Tests Reveal the Best Calcium Supplements
White Plains, New York, May 26, 2017 — Most people know that calcium is important for building and maintaining strong bones, but with so many different forms, dosages and ingredient combinations available on the market, choosing a calcium supplement can be difficult. To help, ConsumerLab.
News Release
February 03, 2017
ConsumerLab.com Shows How Much Choline is Really in Supplements -- ConsumerLab.com Tests Phosphatidylcholine (From Soy Lecithin), Citicoline, Alpha-GPC and Other Choline-Containing Supplements, Identifying Top Picks
White Plains, New York, February 3, 2017 — Choline is an essential nutrient important for liver and brain function. It can be obtained from the diet and most people also produce significant amounts in their bodies.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
June 23, 2020
BioPure Healing Products Warned for Manufacturing Violations
On June 17, 2020, the FDA issued a warning letter to BHP Holdings Inc.
Recalls & Warnings
August 17, 2021
Weight Loss Supplements Found to Contain Sibutramine Recalled
Two companies (including one Ebay seller) recently recalled weight loss supplements that were found to contain sibutramine.
Recalls & Warnings
July 27, 2021
"Miss Slim" Capsules Recalled
On July 20, 2021, HIS issued a recall of all lots and presentations of Miss Slim capsules because FDA analysis found them to contain sibutramine.
Recalls & Warnings
August 16, 2023
FDA Warns Hekma Center, LLC for Promoting Products to Treat Anemia, Diabetes, Depression, & More
On June 2, 2023, the FDA issued a warning letter to Hekma Center, LLC following review of the company’s website and social media, which found statements about the company’s Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa ...
CL Answer
7 Tips to Avoid Getting a Pill Stuck in Your Throat
Some pills can be difficult to swallow and can even become lodged in the throat or cause damage to the esophagus. Learn seven practical tips to help avoid getting pills stuck in the throat.

CL Answer
What are trace minerals and do I need them?
Trace minerals include boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, zinc, and others. Find out what they do, how much of each trace mineral you should be getting, and who's most likely to be deficient in these minerals.
CL Answer
Do any supplements help with restless legs syndrome?
Find out whether supplements or vitamins like iron, magnesium, vitamin E and others can help for restless leg syndrome (RLS). ConsumerLab.com's answer explains.

CL Answer
Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Find out which supplements for nerve pain, including fish oil and alpha-lipoic acid, are effective for sciatica, diabetic neuropathy, or postherpetic neuralgia.

CL Answer
Estrogen Creams for Wrinkles?
Find out if estrogen creams can help reduce wrinkles, whether they are safe, and how "prescription" estriol and estradiol creams and gels differ from those sold over-the-counter.

CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

CL Answer
Here’s What You Should Look For In Toothpaste and Mouthwash, and Our Top Picks
Learn about ingredients you want in your toothpaste, mouthwash and other dental products and which ingredients you may want to avoid.

Recalls & Warnings
April 18, 2022
Moringa Concern: Blood Clots
A 63-year-old woman in New York with type 2 diabetes developed a blood clot in the lungs (i.e. pulmonary embolism) that her physicians suspect may have been caused by the use of a supplement containing Moringa oleifera extract (Ebhohon, Int J Emerg Med 2022).
Recalls & Warnings
June 16, 2020
Seller of Silver, Arginine, and More Warned for Manufacturing Violations
On June 1, 2020, the FDA issued a warning letter to Morningstar Minerals LLC, which found the company's products, including Silver Boost, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
Recalls & Warnings
April 21, 2020
Life Force Warned for Colloidal Silver, Nitric Oxide Claims
On March 27, 2020, the FDA issued a warning letter to Doctor's Signature Sales and Marketing International Corp.
Recalls & Warnings
January 24, 2020
Fat-Burning, Energy Supplement Linked to Heart Trouble
A 33-year-old woman in Australia developed cardiac ischemia (a lack of blood flow to the heart) after taking the "fat burning" supplement Alpha Lean-7, according to a recent report in the Journal of Sports Sciences.
Recalls & Warnings
June 20, 2019
Weight Loss, Muscle & Energy Supplements Linked to Adverse Events in Children and Young Adults
Consumption of dietary supplements sold for weight loss, muscle building, and energy are associated with an increased risk for severe medical events in children and young adults compared to the consumption of vitamins, according to a recent study published in the Journal of the Adolescent ...
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
August 11, 2008
Stroke Attributed to Xenadrine Ephedra-Free Supplement
A case report in the journal Military Medicine (July 2008) attributes the occurance of vasospasm and stroke in a 36 year-old women to use of Xenadrine-EFX, a supplement used for weight loss.
Recalls & Warnings
October 16, 2018
Weight Loss Supplement Containing Hidden Drug Recalled
On October 15, 2018, Fat Burners Zone issued a recall of one lot of its Zero Xtreme weight loss supplement because it was found to contain sibutramine.
Recalls & Warnings
March 19, 2007
Canada Warns of Weight Loss Supplement with Rx Drug
On March 14, 2007, Health Canada advised consumers not to use MIAOZI Slimming Capsules because they have been found to contain sibutramine, a prescription medication that should only be taken under medical supervision.
Recalls & Warnings
March 06, 2019
GoLean Detox Recalled
On February 25, 2019, GoLean Detox USA issued a recall of all lots of GoLean DETOX capsules within expiry because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
January 29, 2019
Prescription Drugs Found in Four Weight Loss Supplements
On January 28, 2019, the FDA the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine and/or phenolphthalein:
Recalls & Warnings
January 22, 2019
Weight Loss Supplement Tainted With Drug
On January 10, 2019, the FDA warned consumers not to use the weight loss supplement Slimina, because it was found to contain sibutramine.
Recalls & Warnings
January 16, 2018
Adverse Effects From Energy Drinks Common Among Youth and Young Adults
More than half (55.4%) of young people who have ever consumed an energy drink have experienced at least one adverse reaction, according to study published yesterday in CMAJ Open, a journal of the Canadian Medical Association.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
December 21, 2012
Dangerous Supplement Being Sold Under New Name
On December 21, 2012, the FDA warned consumers that Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain that has been linked to serious and sometimes fatal side effects, is now being sold under the name WOW.
Recalls & Warnings
October 24, 2012
Manufacturer of Green Tea, Vitamin E, Omega-3 and Cranberry Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On October 16, 2012, the FDA issued a warning letter to dietary supplement manufacturer Advanced Nutritional Technology Inc.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
Recalls & Warnings
September 13, 2010
Recall of Herbal Slimming Supplement Spiked with Drug
The U.S. FDA posted a notice dated August 18, 2010 indicating that Slim-30 Herbal Supplement (Lot 6032101) has been recalled by its distributor, J & H Besta Corp.
Recalls & Warnings
April 23, 2009
Recall of "Slimming" Supplements Spiked with Drug
On April 20, 2009 the U.S. FDA reported a nationwide recall of dietary supplements distributed by Universal ABC Beauty Supply International.
Recalls & Warnings
November 28, 2008
Fat Loss Supplement Recalled for Containing Prescription Drug
On November 26, 2008, the FDA reported that Zhen De Shou Fat Loss Capsules are being recalled by the distributor, Fashion Sanctuary. FDA analysis found the product to contain undeclared sibutramine, an FDA approved drug used as an appetite suppressant for weight loss.
Recalls & Warnings
October 09, 2013
Use of Chinese Supplement Linked to Rare But Serious Condition
On October 9, 2013, Health Canada warned consumers that Compound Danshen Dripping Pills, manufactured by Tianjin Tasly Pharmaceutical Co., have been associated with a case of methemoglobinemia, a rare but serious condition which may result in coma or death.
Recalls & Warnings
November 01, 2017
FDA Warns Sellers of Bodybuilding Supplements Containing Steroid-Like Substances
On October 23, 2017, the FDA issued warning letters to three supplement distributors because several of their bodybuilding products contain selective androgen receptor modulators (SARMs).
Recalls & Warnings
February 27, 2018
Weight Loss Supplement Containing Undeclared Drug Recalled
On February 27, 2018, Bella All Natural recalled their "Diet Capsules" because they contain sibutramine.
Recalls & Warnings
August 08, 2017
Study Shows Severe Risk in Drinking Hydrogen Peroxide
An analysis of records from the National Poison Data System from a 10-year period ending in 2011 identified 294 reported incidents of people developing adverse symptoms after ingesting 35% (high-concentration) hydrogen peroxide (H202). Among these, 41 (13.
Recalls & Warnings
July 26, 2017
Weight Loss Supplements Containing Undeclared Drug Recalled
On July 25, 2017, EZ Weight Loss TX recalled their supplements La Bri's Body Health Atomic and Xplode capsules after FDA analysis found the products to be tainted with sibutramine.
Recalls & Warnings
July 12, 2017
More Bodybuilding Supplements Recalled
On July 11, 2017, Andropharm recalled two bodybuilding supplements, Sten Z and M1 Alpha, because these products contain derivatives of anabolic steroids.
Recalls & Warnings
June 20, 2017
Beware of Bodybuilding Supplements, FDA Warns
On June 20, 2017, the FDA warned consumers to beware of bodybuilding products because a number of these products have been found to contain steroids or steroid-like substances. Such products could cause serious and even life-threatening health risks, including:
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
October 13, 2018
Pharmaceutical Drugs Found In Dietary Supplements Pose Danger to Consumers
Almost 800 dietary supplements sold between 2007 and 2016 contained unapproved pharmaceutical ingredients, according to a study published today in the Journal of the American Medical Association (JAMA).
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
October 30, 2008
Possible Risk Rather than Benefit Found in Trial of Vitamin E and Selenium for Prostate Cancer
On October 27, 2008 the National Cancer Institute (NCI) announced that men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) are being told to stop taking their supplements.
Recalls & Warnings
March 06, 2009
"The Truth About Nutrition" Marketers Agree to Pay $3 Million to Settle Charges of Deceptive Advertising of Dietary Supplements and Devices
On March 6, 2008, the Federal Trade Commission (FTC) announced that marketers of dietary supplements and health-related devices have agreed to pay $3 million in consumer redress to settle FTC charges that they deceptively claimed their products treated or prevented a wide variety of serious ...
Recalls & Warnings
March 13, 2006
FDA Warns About Steroid Products Sold as Dietary Supplements
On March 9, 2006, the U.S.
Recalls & Warnings
July 19, 2010
FDA Warns of Drug in Herbal Slimming Supplement
On July 19, 2010, the U.S. FDA issued a safety warning to consumers regarding Slim-30 Herb Supplement. FDA analysis of the product found it to contain undeclared N-Desmethyl Sibutramine and traces of Sibutramine. Sibutramine is an FDA-approved drug used as an appetite suppressant for weight loss.
Recalls & Warnings
July 09, 2010
FDA Issues Warning on Herbal Weight Loss Supplement
On July 8, 2010, the U.S. Food and Drug Administration (FDA) warned that Que She, marketed as an herbal weight loss supplement, contains active pharmaceutical ingredients not listed on the product label that could harm consumers, especially those with cardiovascular conditions.
Recalls & Warnings
September 12, 2012
Distribution of Weight Loss Product Containing DMAA Should Be Stopped Immediately, Warns FDA
On August 28, 2012, the FDA issued a warning letter to Regeneca stating that the company's RegeneSlim, which was promoted as a dietary supplement for weight loss, is adulterated with dimethylamylamine (DMAA) and that failure to immediately cease distribution of this product could result in FDA ...
Recalls & Warnings
October 24, 2012
Warning Issued to Memory Supplement Maker For Unapproved Drug Ingredient, Drug Claims and Unreported Adverse Events
On October 16, 2012, the FDA issued a letter to dietary supplement manufacturer Quincy Bioscience Manufacturing Inc.
Recalls & Warnings
August 22, 2012
FDA Warns of Life-Threatening Side Effects and Withdrawal Symptoms from Two Arthritis, Muscle Pain Supplements
On August 21, 2012, the FDA issued a warning to consumers about the dangers of taking Reumofan Plus and Reumofan Plus Premium, two dietary supplements marketed for arthritis, osteoporosis and muscle pain.
Recalls & Warnings
July 23, 2009
Weight Loss Supplements Found to Contain Prescription Drug
On July 15, 2009, the U.S. FDA announced that Young You Corporation was recalling four weight loss supplements that contain an undeclared drug ingredient.
Recalls & Warnings
December 08, 2009
Warning on Acai Berry Supplements Spiked with Drug
On December 8, 2009, Health Canada (the Canadian health agency) advised consumers not to use certain Acai Berry products after a large number of shipments of adulterated products were stopped at the border.
Recalls & Warnings
November 17, 2009
Steroids Found in More Body Building Supplements
On November 17, 2009, the U.S.
Recalls & Warnings
November 15, 2009
Nationwide Recall of a Weight Loss Supplement Found to Contain Undeclared Drug Ingredients
On November 12, 2009, The U.S. Food and Drug Administration (FDA) announced that it informed GMP Herbal Products, Inc. that its product "Pai You Guo," a weight loss dietary supplement, contains undeclared drug ingredients.
Recalls & Warnings
October 10, 2014
Weight Loss Supplement Found to Contain Multiple Drugs
On October 10, 2014, the FDA warned consumers not to purchase or use the dietary supplement Japan Hokkaido Slimming Weight Loss Pills because it was found to multiple contain undeclared drugs, including sibutramine, phenolphthalein, benzocaine and diclofenac.
Recalls & Warnings
October 10, 2014
FDA Warns Consumers of Weight Loss Supplement Containing Undeclared Drugs
On October 10, 2014, the FDA warned consumers not to purchase or use the dietary supplement Sit and Slim II because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
September 13, 2014
Three Weight Loss Supplements Found to Contain Drugs
On September 12, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain drugs.
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
August 08, 2013
Weight Loss Supplement Containing Undeclared Drug Recalled
On August 3, 2013, CTV Best Group issued a voluntary recall of all lots of weight loss supplement BEST SLIM because it was found to contain sibutramine.
Recalls & Warnings
August 06, 2013
Weight Loss Supplement Recall Expanded
On August 5, 2013, Bethel Nutritional Consulting, Inc., issued a voluntary recall of herbal weight loss supplements Quick Thin and Bethel Advance because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
July 23, 2013
Weight Loss Supplements Containing Undeclared Drugs Recalled
On July 19, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss supplements Esbelin siloutte te and Esbelin siloutte Herbal Blend with L-Carnitine because they were found to contain undeclared sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
June 30, 2013
Weight Loss Supplements Recalled Due To Undeclared Drug
On May 21, 2013, the Dolphin Intertrade Corp. issued a voluntary recall of one lot of weight loss supplement JaDera and all lots of weight loss supplement Xiyouji Qingzhi, which were found to contain the undeclared drug sibutramine.
Recalls & Warnings
June 27, 2013
Two Weight Loss Supplements Found To Contain Undeclared Drugs
On June 27, 2013, the FDA warned consumers not to use or buy two dietary supplements for weight loss which were found to contain the undeclared sibutramine and/or phenolphthalein.
Recalls & Warnings
June 19, 2013
Weight Loss Supplements Found To Contain Undeclared Drugs, FDA Warns
On June 17, 2013, the FDA advised consumers not to purchase or use the weight loss supplements listed because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein.
Recalls & Warnings
June 14, 2013
Weight Loss Supplement Found To Contain Undeclared Drug
On June 6, 2013, the FDA warned consumers that weight loss dietary supplement XIYOUJI QINGZHI CAPSULE was found to contain undeclared simbutrimine.
Recalls & Warnings
June 12, 2013
Weight Loss Supplement Recalled Due To Undeclared Drugs
On June 11, 2013, Bethel Nutritional Consulting, Inc. issued a voluntary recall of one lot of Bethel 30 herbal weight loss supplement because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
May 29, 2013
Seller of Colloidal Silver, Noni Juice and Noni Supplements Warned For Drug Claims
On May 16, 2013, the FDA issued a warning letter to Matrix Health Products, Inc.
Recalls & Warnings
July 28, 2013
B Vitamin Supplement Found To Contain Anabolic Steroids
On July 26, 2013, the FDA warned consumers not to purchase or use Healthy Life Chemistry by Purity First B-50, a vitamin B dietary supplement which was found to contain anabolic steroids, methasterone and dimethazine.
Recalls & Warnings
April 04, 2013
FDA Warns Consumers Of Weight Loss Supplement Containing Undeclared Drug
On April 4, 2013, the FDA warned consumers not to purchase or use the dietary supplement MAXILOSS Weight Advanced Blue because it was found to contain sibutramine.
Recalls & Warnings
February 25, 2013
Weight Loss Supplement Recalled Due To Undeclared Drug
On February 21, 2013, Olaax Corp. issued a voluntary, nationwide recall of the company's weight loss supplement MAXILOSS Weight Advanced softgels because they were found to contain undeclared Sibutramine.
Recalls & Warnings
February 20, 2013
Recall: Arthritis and Muscle Pain Supplement Contains Prescription Drugs
On February 15, 2013, Reumofan Plus USA, LLC and Reumofan USA, LLC announced a recall of Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain, because it was found to contain the active pharmaceutical ingredients methocarbamol, dexamethasone, and diclofenac.
Recalls & Warnings
February 15, 2013
FDA Seizes Weight Loss Supplements Containing Undeclared Drug
On Feb. 14, 2013, U.S. Marshals acting on behalf of the FDA seized weight loss supplements from Globe All Wellness, LLC which were found to contain the undeclared drug sibutramine hydrochloride.
Recalls & Warnings
March 08, 2012
Rx Drugs Found in Weight Loss and Enhancement Supplements
On February 6, 2012, FDA issued a letter to Globe All Wellness, LLC warning that two of their products contain undeclared prescription drugs.
Recalls & Warnings
March 03, 2012
Seller of Cancer Cures Warned by FDA
On February 9, 2012, the U.S. FDA sent a Warning Letter to BioAnue Laboratories, Inc. informing it that its websites at www.tumorx.com, www.cancerx.org, www.hopewelltechnologieslimited.com, and www.vmhe.
Recalls & Warnings
February 22, 2012
"Japan Weight Loss Blue" Pills Dangerous According to FDA
On February 18, 2012, the U.S. Food and Drug Administration (FDA) advised consumers not to purchase or use “Japan Weight Loss Blue,” a product for weight loss sold on various websites, including www.vitaminbestbuy.com.
Recalls & Warnings
February 07, 2012
Drug-Laced Supplements and Drink Mixes Recalled
According to the U.S. FDA, on February 3, 2012 Healthy People Co. announced a voluntary nationwide recall of several dietary supplements sold as capusules, drinks, and shake mixes. The supplements were found to contain drug substances and pose risks.
Recalls & Warnings
August 07, 2011
Performance Enhancing Ingredient, DMAA, Not Really from Geraniums, Putting Its Use in Supplements in Doubt
On July 29, 2011, the American Herbal Products Association (AHPA) announced a new requirement that products containing the sports performance enhancing compound DMAA (also known as 1,3-dimethlypentlyamine, 1,3 dimethylamylamine, methylhexanamine, or MHA), not be labeled as geranium oil or any part ...
Recalls & Warnings
July 19, 2011
FDA Warns of Unsafe Drug in Slimming Supplements
On July 8, 2011, the U.S. FDA advised consumers not to purchase or use Slim Forte Slimming Capsule and Slim Forte Double Power Slimming Capsules. FDA laboratory analysis confirmed that these products contain sibutramine. Sibutramine is a controlled substance that was removed from the U.S.
Recalls & Warnings
February 25, 2011
Weight Supplement Found by FDA to Contain Prescription Drug
The U.S. FDA posted an announcement (dated February 9, 2011) by Svelte 30 Nutritional Consultants indicating that a sample of Svelte 30 orange & gray capsule was collected and tested by FDA in January 2011.
Recalls & Warnings
February 24, 2011
Recall of Counterfeit Extenze Tablets Containing Drugs
On February 23, 2011, the FDA announced that Biotab Nutraceuticals, Inc. was notified that two lots of counterfeit product purporting to be Extenze contain undeclared drug ingredients. Specifically, lot 0709241 contains tadalafil and sildenafil, and lot 0509075 contains tadalafil and sibutramine.
Recalls & Warnings
January 04, 2011
Weight Loss Supplement Containing a Drug Can Cause Serious Adverse Events, Warns FDA
On December 31, 2010 the U.S. FDA warned consumers not to use Fruta Planta weight loss products because they contain sibutramine, a drug withdrawn from the market in December 2010 for safety reasons.
Recalls & Warnings
October 08, 2010
FDA Warns of Stimulant in Slimming Capsules
On October 8, 2010, the U.S. FDA advised consumers who have Slimming Beauty Bitter Orange Slimming Capsules not to use the product. FDA warns that Slimming Beauty Bitter Orange Slimming Capsules contain the active pharmaceutical ingredient sibutramine, a prescription-only drug which is a stimulant.
Recalls & Warnings
March 13, 2015
Weight Supplement Containing Drugs Recalled
On March 10, 2015, UltraZx, Labs, L.L.C, issued a voluntary recall of all lots of weight loss supplement UltraZx because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
March 06, 2015
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement Containing Hidden Drug
On March 5, 2015, the FDA warned consumers not to buy or use weight loss supplement L-Carnitine Sob Strengthening Version Slimming Miracle Capsule because it was found to contain sibutramine.
Recalls & Warnings
March 03, 2015
Weight Supplement Contains Hidden Drug
On March 2, 2015, the FDA warned consumers not to buy or use weight loss supplement Elimulating Weight & Toxin Keeping Beauty because it was found to contain sibutramine.
Recalls & Warnings
February 26, 2015
Weight Loss Supplement Found to Contain Multiple Drugs
On February 25, 2015, the FDA warned consumers not to buy or use weight loss supplement Lean Body Extreme because it was found to contain sibutramine, desmethyl sibutramine, phenolphthalein, and sildenafil.
Recalls & Warnings
January 06, 2015
FDA Warns of Weight Loss Supplement Dangers
On January 5, 2015, the FDA warned consumers that many weight loss products, including supplements, coffees and teas, may promise results they cannot deliver, or contain dangerous hidden drugs.
Recalls & Warnings
December 20, 2014
Weight Loss Supplement Recalled
On December 19, 2014, Bethel Nutritional Consulting, Inc. issued a recall of one lot of weight loss supplement SLIM-K Capsules because they were found to contain sibutramine.
Recalls & Warnings
November 26, 2014
Weight Loss Supplement Found to Contain Hidden Drug
On November 25, 2014, the FDA warned consumers not to buy or use weight loss supplement Slim Vie because it was found to contain sibutramine.
Recalls & Warnings
November 25, 2014
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On November 24, 2014, the FDA warned consumers not to buy or use weight loss supplement Bee Slim because it was found to contain sibutramine.
Recalls & Warnings
November 24, 2014
Weight Loss Supplement Contains Hidden Drug
On November 24, 2014 the FDA warned consumers not to buy or use weight loss supplement Super Extreme Accelerator because it was found to contain sibutramine.
Recalls & Warnings
November 21, 2014
Weight Loss Supplements Recalled
On November 19, 2014, REFA Enterprises, LLC issued a voluntary recall of one lot each of Forever Beautiful Bee Pollen and Forever Beautiful Infinity because they were found to contain sibutramine or a combination of sibutramine and phenolphthalein.
Recalls & Warnings
November 05, 2014
Slimming Coffee Found to Contain Drug
On November 4, 2014, the FDA warned consumers not to purchase or use the dietary supplement V26 Slimming Coffee because it was found to contain sibutramine.
Recalls & Warnings
June 20, 2014
FDA Warns Some Bee Pollen Products for Weight Loss Are Dangerous
On June 19, 2014, the FDA warned consumers that some weight loss products containing bee pollen have been found to contain hidden drugs.
Recalls & Warnings
June 18, 2014
Two Weight Loss Supplements Found to Contain Drugs
On June 17, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain drugs.
Recalls & Warnings
June 11, 2014
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On June 10, 2014, the FDA warned consumers not to buy or use weight loss supplement La Jiao Shou Shen because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
June 05, 2014
FDA Warns Consumers Not to Buy Use Weight Loss Supplement Containing Hidden Drugs
On June 5, 2014, the FDA warned consumers not to buy or use weight loss supplement B-Perfect because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
May 16, 2014
Weight Loss Supplement Found To Contain Drug
On May 16, 2014, the FDA warned consumers not to buy or use weight loss supplement Asset Bold because it was found to contain sibutramine.
Recalls & Warnings
May 15, 2014
FDA Warns Consumers Not To Buy Or Use Weight Loss Supplement
On May 12, 2014, the FDA warned consumers not to buy or use weight loss supplement Asset Bee Pollen because it was found to contain sibutramine.
Recalls & Warnings
May 06, 2014
Two Weight Loss Supplements Found To Contain Drug
On May 5, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine.
Recalls & Warnings
May 01, 2014
Weight Loss Supplement Recalled
On April 29, 2014, dietary supplement distributor Bacai Inc. issued a voluntary recall of one lot of weight loss supplement LiteFit USA because it was found to contain sibutramine.
Recalls & Warnings
April 18, 2014
Weight Loss Supplement Recalled
On April 9, 2014, Nature's Universe issued a voluntary recall of all lots of Thinogenics weight loss capsules sold prior to February 6, 2014 because they were found to contain sibutramine.
Recalls & Warnings
April 11, 2014
Two Weight Loss Supplements Found to Contain Drug
On April 10, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine.
Recalls & Warnings
April 03, 2014
Weight Loss Supplement Containing Drugs Recalled
On April 2, 2014, the FDA warned consumers not to buy or use weight loss supplement New You because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
March 27, 2014
Bee Pollen and Weight Loss Supplements Recalled
On March 26, 2014, Pure Edge Nutrition, LLC issued a voluntary recall of Bella Vi Insane Bee Pollen Capsules, Bella Vi BTrim Ultimate Boost, Bella Vi BTrim Max, Bella Vi Extreme Accelerator, Bella Vi Insane Amp'd, and two lots of Bella Vi Amp'd Up because they were found to contain sibutramine, ...
Recalls & Warnings
March 27, 2014
Three Weight Loss Supplements Containing Drugs Recalled
On March 25, 2014, New Life Nutritional Center issued a voluntary recall of all lots of Super Fat Burner capsules, Maxi Gold capsules and Esmeralda softgels because they were found to contain sibutramine, phenolphthalein or a combination of both drugs.
Recalls & Warnings
March 19, 2014
Weight Loss "Coffee" Found to Contain Drug
On March 19, 2014, the FDA warned consumers not to buy or use Vitaccino Coffee because it contains sibutramine.
Recalls & Warnings
February 28, 2014
Weight Loss Supplement Contains Undeclared Drug
On February 18, 2014, Health Canada warned consumers not to use weight loss supplement LV Shou Reduces Fat because it contains sibutramine.
Recalls & Warnings
February 17, 2014
Weight Loss Supplement Recall Expanded to Include Additional Products
On February 4, 2014, MyNicKnaxs, LLC, which first issued a recall of Reduce Weight Fruta Planta, issued a recall of nine additional weight loss supplements: Magic Slim, Fruta Bio, SlimEasy, Super Fat Burning Bomb, Slim Xtreme, Meizi Evolution, Meizitang Strong Version Botanical Slimming, ...
Recalls & Warnings
January 30, 2014
Four Weight Loss Supplements Found To Contain Drugs
On January 28, 2014, the FDA warned consumers not to buy or use the four weight loss supplements listed below because they were found to contain sibutramine or phenolphthalein.
Recalls & Warnings
January 23, 2014
"Slimming" Supplements Found to Contain Drugs
On January 21, 2014, the FDA advised consumers not to buy or use weight loss supplements Dream Body Slimming Capsule and Magic Slim because they were found to contain sibutramine and/or phenolphthalein.
Recalls & Warnings
December 26, 2013
Weight Loss Supplement Recalled
On December 23, 2013, Deseo Rebajar Inc. issued a voluntary recall of one lot of Burn 7 Capsules because they were found to contain sibutramine.
Recalls & Warnings
December 24, 2013
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On December 19, 2013, the FDA warned consumers not to buy or use weight loss supplement Dr. Ming's Chinese Capsule because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
December 24, 2013
Muscle Growth Supplement Linked with Liver Failure
On December 23, 2013, the FDA advised consumers to immediately stop using muscle growth supplement Mass Destruction (by Blunt Force Nutrition) because it is labeled as containing at least one synthetic anabolic steroid and has been linked to at least one reported case of liver failure.
Recalls & Warnings
December 20, 2013
Five Weight Loss Supplements Found to Contain Undeclared Drug
On December 19, 2013, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared sibutramine.
Recalls & Warnings
November 21, 2013
FDA Warns of Five More Weight Loss Supplements Containing Undeclared Drugs
On November 21, 2013, the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein:
Recalls & Warnings
November 20, 2013
Weight Loss Supplement Found to Contain Drugs
On November 19, 2013, the FDA warned consumers not to buy or use weight loss supplement Slim Max because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
November 12, 2013
Weight Loss Supplement Found To Contain Drugs
On November 8, 2013, Health Canada warned consumers of a number of weight loss products that have been found to contain sibutramine or phenolphthalein, including Paiyouji Natural Slimming Capsules, which may be available for purchase to U.S. consumers through online retailers.
Recalls & Warnings
November 07, 2013
FDA Warns Consumers of Weight Loss Supplement Containing Multiple Drugs
On November 7, 2013, the FDA warned consumers not to buy or use Jimpness Beauty Fat Loss Capsules because they were found to contain sibutramine, phenolphthalein, and sildenafil.
Recalls & Warnings
November 06, 2013
Weight Loss Supplement Found to Contain Drugs
On November 5, 2013, the FDA advised consumers not to purchase or use the weight loss supplement Goodliness Fat-Reducing capsules, which were sold on various websites, because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
October 27, 2013
Ingredient In Workout Supplements May Be Unsafe, FDA Warns
On October 11, 2013, the FDA directed USP Labs, LLC to cease distribution of workout supplement OxyElite Pro and and muscle-building supplement VERSA-1, which are labeled as containing the ingredient aegeline, or N-[2-hydroxy-2(4-methoxyphenyl) ethyl]-3-phenyl-2-propenamide.
Recalls & Warnings
October 16, 2013
Methamphetamine-Like Compound Found In Popular Workout Supplement
A study published on October 14, 2013 (Cohen, Drug Test Analysis 2013) reports that the popular pre-workout energy supplement Craze (Driven Sports) has been found to contain the banned methamphetamine — like compound, N, á-diethylphenylethylamine.
Recalls & Warnings
October 10, 2013
Six Weight Loss Supplements Found to Contain Drugs
On October 10, 2013, the FDA advised consumers not to purchase or use the six weight loss supplements listed below because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein.
Recalls & Warnings
November 08, 2016
Weight Loss Supplements Contain Hidden Drugs, FDA Warns
The FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared drugs:
Recalls & Warnings
November 21, 2015
Ultimate Herbal Slimcaps Recalled
On November 19, 2015 Fit Firm and Fabulous issued a voluntary recall certain lots of weight loss supplement Ultimate Herbal Slimcap capsules because they were found to contain sibutramine.
Recalls & Warnings
November 10, 2015
"Natureal" Weight Supplement Recalled
On November 9, 2015 Inaffit, LLC issued a voluntary recall of all lots of weight loss supplement Natureal because it was found to contain undeclared sibutramine.
Recalls & Warnings
October 24, 2015
Four Weight Loss Supplements Found To Contain Drugs
On October 23, 2015, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine, phenolphthalein and/or sildenfil. Each was identified during an examination of international mail shipments:
Recalls & Warnings
October 02, 2015
Drug Found in Two Weight Loss Supplements
On October 1, 2015 the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine:
Recalls & Warnings
September 09, 2015
Weight Supplement Found to Contain Drug
On September 3, 2015 the FDA warned consumers not to buy or use the weight loss supplement Meizi Super Power Fruits Herbal Slimming Formula because it was found to contain sibutramine.
Recalls & Warnings
August 07, 2015
Weight Loss Supplement Found to Contain Drug
On August 6, 2015 the FDA warned consumers not to buy or use the weight loss supplement Achieving Zero because it was found to contain sibutramine.
Recalls & Warnings
July 25, 2015
Weight Supplement Found to Contain Three Drugs
On July 23, 2015 Life & More, L.L.C. issued a voluntary recall of 783 bottles from one lot of Akttive High Performance Fat Burner Gold weight loss capsules because they were found to contain the undeclared drugs sibutramine, desmethylsibutramine, and phenolphthalein.
Recalls & Warnings
July 02, 2015
Undeclared Drugs Found in Two Weight Loss Supplements
On July 2, 2015 the FDA warned consumers not to buy or use weight loss supplements listed below because they were found to contain undeclared drugs. Click on the name of each product to read the full warning.
Recalls & Warnings
June 06, 2015
Weight Supplement Recalled
On June 3, 2015 SmartLipo365 issued a voluntary recall of 122 lots of Smart Lipo because they were found to contain the undeclared drugs sibutramine, desmethylsibutramine, and phenolphthalein.
Recalls & Warnings
May 02, 2015
Drug Found in Two Weight Loss Supplements
The FDA recently warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine.
Recalls & Warnings
April 14, 2015
Muscle Supplement Linked to Serious Liver Injury
On April 13, 2015, the FDA warned consumers not to use the muscle growth supplement Tri-Methyl Xtreme (distributed by Extreme Products Group) because it has been linked to serious liver injury.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
March 13, 2015
Weight Loss Supplement Contains Prescription Anti-Depressant, Other Drugs
On March 3, 2015, the FDA warned consumers not to buy or use weight loss supplement Natural Max Slimming because it was found to contain fluoxetine, sildenafil and sibutramine.
Recalls & Warnings
August 20, 2016
Smart Lipo Weight Loss Supplement Found to Contain Undeclared Drugs
On June 16, 2016, the FDA issued a warning letter to Centro Naturista because the company's weight loss supplement, Smart Lipo, was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
July 03, 2016
Weight Loss Supplement Recalled
On June 1, 2016, Dream Body Weight Loss issued a recall of all lots of the following weight loss supplements listed because they were found to contain undeclared sibutramine:
- Dream Body Extreme Gold 800 mg 30 gold capsules
Recalls & Warnings
June 07, 2016
Step 2 Weight Loss Supplement Recalled
On June 7, 2016, The Body Shot Bar issued a recall of all lots of weight loss supplement Step 2 60 gold capsule (350 mg per) capsules which were distributed between March 1 through May 6 2016 because they were found to contain undeclared sibutramine.
Recalls & Warnings
May 07, 2016
Weight Loss Supplement Contains Hidden Drug, FDA Warns
On May 6, 2016 the FDA warned consumers not to buy or use weight loss supplement Step 2 because it was found to contain undeclared sibutramine.
Recalls & Warnings
May 02, 2016
Weight Loss Supplements Containing Hidden Drugs Recalled
On April 29, 2016 Making It A Lifestyle, L.L.C. issued a recall of all lots of weight loss supplements 3rd Degree, Black Gold X Advanced and Black Label X because they were found to contain undeclared sibutramine and sildenafil.
Recalls & Warnings
April 13, 2016
"Super Herbs" Weight Loss Supplement Recalled
On April 11, 2016 Super Herbs issued a recall of all lots of weight loss supplement SUPER HERBS because it was found to contain sibutramine, desmethylsibutramine, and/or phenolphthalein.
Recalls & Warnings
April 02, 2016
Propell Platinum Contains Undeclared Drugs
On March 29, 2016, the FDA warned consumers not to buy or use the weight loss supplement Propell Platinum because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
March 29, 2016
Weight Loss Supplement Envy BP Contains Undeclared Drug
On March 28, 2016, the FDA warned consumers not to buy or use the weight loss supplement ENVY BP because it was found to contain undeclared sibutramine.
Recalls & Warnings
March 20, 2016
FDA Warns of Weight Supplements Containing Undeclared Drugs
On March 17, 2016, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared drugs:
Recalls & Warnings
January 31, 2016
Pink Bikini and Shorts on the Beach Weight Supplements Recalled
On January 28, 2015, Lucy's Weight Loss System issued a voluntary recall of all lots of weight loss supplements Pink Bikini (white capsules, blue capsules and gold capsules) and Shorts on the Beach (blue capsules and gold capsules) because they contain undeclared sibutramine, ...
Recalls & Warnings
December 23, 2015
"Bee Extremely Amazed" Recalls Weight Supplements
On December 22, 2015, Bee Extremely Amazed LLC issued a voluntary recall of all lots of the following weight loss supplements, which were found to contain sibutramine and phenolphthalein:
Recalls & Warnings
December 19, 2015
Don't Get Stung by Bee Pollen
On December 18, 2015, the FDA warned consumers not to buy or use the following supplments promoted for weight loss because they were found to contain sibutramine and phenolphthalein:
Recalls & Warnings
December 19, 2015
"Thirty Plus" Contains Hidden Drug
On December 18, 2015, the FDA warned consumers not to buy or use the weight loss supplement Thirty Plus because it was found to contain sibutramine.
Recalls & Warnings
December 19, 2015
Drugs Found in Jenesis Weight Supplement
On December 18, 2015, the FDA warned consumers not to buy or use the weight loss supplement Jenesis because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
December 05, 2015
Lipo Escultura Weight Loss Capsules Recalled
On December 3, 2015 Lipo Escultura Corp. (doing business as JAT Productos Naturales Corp.) issued a voluntary recall of weight loss supplement Lipo Escultura capsules because they were found to contain sibutramine and diclofenac.
Recalls & Warnings
July 10, 2017
Bodybuilding Supplements Containing Steroid-Like Substances Recalled
On June 29, 2017, Hardcore Formulations recalled all lots and expiration dates of their products Ultra-Sten and D-Zine, bodybuilding supplements found to contain methylstenbolone and dymethazine, which are derivatives of anabolic steroids.
Recalls & Warnings
July 29, 2017
Increase in Calls to Poison Control Centers About Supplements
The number of calls to poison control centers in the U.S. about dietary supplement exposures increased by almost 50% between 2005 and 2012, according to a study published this week in the Journal of Medical Toxicology.
Recalls & Warnings
October 07, 2017
Weight Loss Supplement Recalled
On October 5, 2017, Kiriko, LLC recalled recalled all lots of A1 Slim 30 capsules after FDA analysis found the products to contain sibutramine, phenolphthalein and N-Desmethyl sibutramine.
Recalls & Warnings
May 26, 2017
"Allergy" Supplement Containing Ephedra Recalled
On February 7, 2017, MusclMasster, LLC (DBA The Green Herb) of Wheat Ridge, CO issued a recall of all bottles of Al-Er-G Capsules , a product promoted to help allergies, because they contain ephedra.
Recalls & Warnings
May 20, 2017
Muscle Enhancement Supplement Containing Steroid-Like Substances Recalled
On May 19, 2017, Dynamic Technical Formulations LLC issued a recall of all lots its muscle enhancement supplement Tri-Ton because it was found to contain anabolic steroid-like substances (andarine and ostarine) which are not permitted in dietary supplements.
Recalls & Warnings
May 08, 2017
Muscle Enhancement Supplement Recalled
On May 5, 2017, Genetic Edge Compounds issued a recall of all lots its muscle enhancement supplement GEC Laxoplex because FDA analysis found it to contain anabolic steroids and steroid like substances, which are not permitted in dietary supplements.
Recalls & Warnings
April 01, 2017
Weight Loss Supplement Containing Undeclared Drug Recalled
On March 28, 2017, Envy Me issued a recall of weight loss supplement LaBri's Body Health Atomic because it was found to contain undeclared sibutramine. Sibutramine, the active ingredient in the obesity drug Meridia, was removed from the U.S.
Recalls & Warnings
February 09, 2017
Herbal Supplement Found to Contain Ephedra Alkaloids Recalled
On February 7, 2017, Kingsway Trading Inc. issued a recall of Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains ephedra alkaloids.
Recalls & Warnings
February 04, 2017
Five Weight Loss Supplements Found to Contain Drugs
The FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs:
Recalls & Warnings
January 24, 2017
Weight Loss Supplements Found to Contain Drugs
The FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs:
Recalls & Warnings
December 23, 2016
Weight Loss Supplement Found to Contain Antidepressant
On December 22, 2016, the FDA warned consumers not to buy or use the weight loss supplement Queen Slimming Soft Gel because it was found to contain undeclared fluoxetine and sibutramine.
Recalls & Warnings
February 13, 2018
American College of Sports Medicine Warns of Energy Drink Dangers
A new statement released by the American College of Sports Medicine (ACSM) highlights the dangers of energy drinks when consumed by children and adolescents, and calls for more studies on the safety and efficacy energy drinks.
Recalls & Warnings
December 01, 2015
Weight Supplements Found to Contain Antidepressant and Other Prescription Drugs
On November 19, 2015, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain prescription drugs. Each was identified during an examination of international mail shipments:
Recalls & Warnings
March 13, 2015
Three Weight Loss Supplements Found to Contain Drugs
The FDA recently warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
August 07, 2015
Male Enhancement and Weight Loss Supplement Containing Drugs Recalled
On August 6, 2015 Blue Square Market Inc. issued a recall of the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
November 01, 2016
Eleven Weight Loss Supplements Contain Undeclared Drugs, FDA Warns
The FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared drugs:
Recalls & Warnings
April 16, 2014
Weight Loss Supplements Found To Contain Prescription Antidepressant and Other Drugs
On March 7, 2014, the FDA issued a warning letter to Deseo Rebajar Inc. because the company's following weight loss products were found to contain drugs:
Recalls & Warnings
February 01, 2013
DMAA Supplement Linked to Runner's Death
The cause of death of London marathon runner Claire Squires has been ruled by the investigating coroner as cardiac failure due to extreme exertion, complicated by DMAA toxicity.
Recalls & Warnings
March 29, 2013
Prescription Drugs Found In Prostate and Sexual Enhancement Supplements
October 24, 2012 the FDA issued a warning letter to USA Far Ocean Group Inc./ Health & Beauty Group Inc. because a number of the company's dietary supplements were found to contain pharmaceutical drugs, or were promoted with statements that constitute drug claims.
Recalls & Warnings
July 26, 2013
Safety of Certain Weight Loss and Bodybuilding Supplements Called Into Question
On July 25, 2013, USA Today reported that several supplements, including weight loss, body building and sports nutrition supplements, have come under scrutiny after being found to contain illegal ingredients, or were associated with failed drug tests and adverse events.
Recalls & Warnings
August 02, 2013
Vitamin B, Vitamin C and Multimineral Supplements Recalled Due To Anabolic Steroid Risk
On July 31, 2013, Purity First Health Products, Inc.
Recalls & Warnings
August 20, 2013
Weight Loss Supplement Formulas For Men and Women Recalled Due To Undeclared Drugs
On August 16, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss dietary supplements Esbelder man, Esbelder fem and Esbelder siloutte because they were found to contain the undeclared drugs sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
August 02, 2014
Judge Orders BioAnue To Stop Illegal Cancer and Disease Claims
On July 23, 2014, Gloria D.
Recalls & Warnings
July 10, 2014
Five Weight Loss Supplements Found to Contain Undeclared Drugs
On July 8, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared sibutramine and/or phenolphthalein.
Recalls & Warnings
November 03, 2009
Recall of 65 Dietary Supplements That May Contain Steroids
On November 3, 2009, as part of its ongoing cooperation with the Food and Drug Administration ("FDA"), Bodybuilding.
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
January 16, 2010
Recall of Body Building Supplements Containing Steroids
On January 15, 2010, the Food and Drug Administration (FDA) posted a voluntary recall notice from MuscleMaster.com, Inc. regarding all lots and expiration dates of seventeen dietary supplements sold in 2009 from June 1 and Novemember 17.
Recalls & Warnings
March 03, 2006
FDA Requests Seizure of More Supplements Containing Ephedrine Alkaloids
On February 24, 2006, the U.S. Food and Drug Administration (FDA) announced that the U.S.
Recalls & Warnings
January 05, 2005
Marketers of Brain-Rejuvinating Supplement Settle FTC Charges of False Claims
On January 5, 2005, the U.S. Federal Trade Commission (FTC) announced that a company that heavily advertised an herbal dietary supplement called "Sagee" to the Chinese-language and Vietnamese-language communities had settled FTC charges of making false and unsubstantiated claims for the product.
Recalls & Warnings
January 03, 2004
FDA to Prohibit Sales of Dietary Supplements Containing Ephedra
On December 30, 2003, the U.S. Food and Drug Administration (FDA) issued a consumer alert of its forthcoming determination that dietary supplements containing ephedra present an unreasonable risk of illness or injury, and should not be consumed.
Recalls & Warnings
December 22, 2008
FDA Warns Consumers About Tainted Weight Loss Pills
On December 22, 2008 the U.S. FDA alerted consumers not to purchase or consume any of more than 25 different products marketed for weight loss because they contain undeclared, active pharmaceutical ingredients that may put consumers’ health at risk.
Recalls & Warnings
August 16, 2006
Marketers of Diabetes Supplement Banned From Claiming Products Treat or Cure Diseases
On August 10, 2006, the Federal Trade Commission (FTC) announced that an operation selling Chinese herbal supplements is banned from claiming its products treat or cure diseases, to settle FTC charges it violated a previous court order.
Recalls & Warnings
March 04, 2003
U.S. Warns of Ephedra Risks and Proposes Warning Label for Supplements
On February 28, the Department of Health and Human Services (HHS) announced a series of actions designed to protect Americans from potentially serious risks of dietary supplement products containing ephedra.
News Release
October 14, 2015
Is Matcha a Better Form of Green Tea? ConsumerLab.com Answers the Question
White Plains, New York, October 14, 2015 — For the past three years, ConsumerLab.com has been testing green tea products, including tea bags, bottled drinks, dietary supplements, and even a K-Cup®.
News Release
August 28, 2015
Not All Vitamin E Supplements Are Alike, Especially "Natural" Ones, ConsumerLab.com Tests Reveal
White Plains, New York, August 28, 2015 — ConsumerLab.com recently tested popular brands of natural and synthetic vitamin E supplements and found that not all vitamin E supplements are the same - with big differences in the types and amounts of compounds they contain.
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
March 19, 2004
Seasilver Stopped from Making Claim to Cure "Over 650" Diseases
On March 17, the Food and Drug Administration (FDA) announced that Seasilver USA, Inc., and Americaloe, Inc.
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...
News Release
June 18, 2012
What is "Natural" Vitamin E? It Depends On the Brand Selling It, Finds ConsumerLab.com -- New Review of Vitamin E Supplements, Oil and Cream, Including Tocopherols and Tocotrienols
White Plains, New York, June 18, 2012 — What is in a "natural" vitamin E supplement, cream, or oil? According to recent tests by independent evaluator, ConsumerLab.com, this depends completely on the brand selling it.
News Release
July 01, 2008
Tests of ten red yeast rice supplements by ConsumerLab.com reveal significant statin levels, but some pills contaminated -- Popular cholesterol-lowering supplements compared in new report
WHITE PLAINS, NEW YORK — JULY 1, 2008 — ConsumerLab.
News Release
September 10, 2007
Tests of B-vitamins by ConsumerLab.com finds several short on folic acid — New report covers B-complexes, thiamin, niacin, B-6, B-12, biotin and folic acid supplements
WHITE PLAINS, NEW YORK — SEPTEMBER 10, 2007 — Half of the B-complex supplements recently selected by ConsumerLab.com for testing were found to lack the total claimed amounts of folic acid, an essential nutrient also known as vitamin B-9.
News Release
March 30, 2005
ConsumerLab.com finds most B-vitamins of high quality but three lacking in ingredients — Test Results for 41 Products Reported Along with Information on Use
WHITE PLAINS, NY — MARCH 30, 2005 — ConsumerLab.com reported test results today for B vitamin supplements that it recently purchased in the U.S. and Canada.





