Reviews and Information for Stimulant Blends
Weight Loss Supplements (7-Keto DHEA, Forskolin and Stimulant Blend Supplements)
Trying to find the best and safest weight loss supplements? See our independent tests of popular brands and our review of the evidence for ingredients like 7-keto DHEA, forskolin, and stimulant blends containing sources of caffeine and synephrine. Learn about efficacy, dosage, side effects, and potential drug interactions.
Weight Loss Supplements (7-Keto DHEA, Forskolin and Stimulant Blend Supplements) ReviewSearch term may appear only in full report available to members. Join now for full access.
Product Review
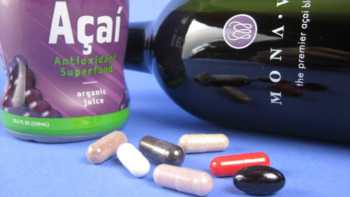
Acai Berry Supplements and Beverages Review
Find the Best Acai Berry Supplements and Beverages. See Which Acai Berry Supplements and Beverages Passed Our Tests of Quality.
CL Answer
Can zuPOO and its primary ingredient, cascara sagrada, cleanse the gut, improve digestion and help with weight management?
zuPOO is marketed for gut support and as a colon cleanser for weight management. Find out if research supports these claims and if ingredients in zuPOO are safe.

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

CL Answer
What is the difference between cocoa and cacao? Is one better than the other?
Learn about the difference between cocoa vs. cacao, including cocoa powder, cocoa extracts, cocoa liquor, and cacao nibs. Information about benefits, caffeine, theobromine in cocoa and cacoa, differences between dark chocolate and milk chocolate. ConsumerLab.com's answer explains.

Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Product Review
Olive Leaf Extract Supplements Review
Learn What Olive Leaf Does and the Best Brands

Product Review
Lion's Mane and Chaga Supplements Review
Read Labels Carefully -- Many Can Mislead

Product Review
Nitric Oxide Supplements (Bodybuilding and Athletic Performance) Review Article
Do Nitric Oxide Supplements Really Work? Find Out If Nitric Oxide Supplements Really Build Muscle or Improve Performance.

CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

Product Review
CLA (Conjugated Linoleic Acid) Supplements Review (for Slimming)
Choose the Best CLA Supplement. Not All CLA Supplements Contain What You Expect.

Product Review
Prebiotic Supplements Review
You Can't Tell How Much Fiber Is Really In Most Prebiotics Without Testing

News Release
November 02, 2023
Spirulina and “Greens” Supplement Tests Show Some Problems
White Plains, New York, November 2, 2023 — ConsumerLab tests of spirulina, chlorella, and “greens” supplements revealed that tablets of three brands of spirulina failed to disintegrate within the allotted time, and several other products contained amounts of lead unsuitable for regular consumption ...
Product Review
Lavender and Tea Tree Essential Oils Review
Oils Tested for Authenticity and Purity

CL Answer
Is the theobromine in chocolate and cocoa good or bad for me?
Find out the benefits and risks of theobromine -- and how much is in dark chocolate.

Recalls & Warnings
May 09, 2022
Natural Organics Keto Capsules Recalled Due to Gluten
On March 6, 2022, Natural Organics, Inc. issued a recall of four lots of NaturePlus Keto Living Sugar Control Capsules that were found to contain gluten.
CL Answer
What is CELLFOOD and is it a healthy supplement?
Learn more about CELLFOOD (NuScience Corporation), including if the claims to improve athletic performance, fibromyalgia, weight loss, and cancer are true.
CL Answer
Does AZO Bladder Control really work for overactive bladder?
AZO Bladder Control information, including results from clinical studies on bladder control, dosage, and safety.

News Release
April 10, 2023
Best and Worst Olive Oils According to ConsumerLab Tests
White Plains, New York, April 10, 2023 — Extra virgin olive oil can be a heart-healthy alternative to saturated fats in the diet, providing antioxidant polyphenols and plenty of oleic acid, a “healthy” monounsaturated fat.
Clinical Update
6/16/2013
Stimulant Supplements
Have you ever wondered what taking a stimulant supplement for weight management or energy might do to your body? Researchers recently gave Zantrex-3, Xenedrine EFX, Metabolift, or a brand of Guarana to young men while monitoring their heart rates and other vital signs, and recording side effects. The results raise concerns. Get the details, plus test results for 14 other supplements, in the updated Weight Supplements Review >>
CL Answer
Conolidine: Evidence for Pain Relief, Safety, and CONOCB2™ Products Explained
Find out if conolidine, the main ingredient in CONOCB2 Blend, offers natural pain relief without the side effects of opioids. Also learn about the top conolidine products available today.

CL Answer
Have you heard of the probiotic, Keybiotics? Does it do what it claims, and is it worth the money they charge?
Whole Body Research Keybiotics probiotic information, including its ingredients and possible cheaper alternatives.

News Release
February 09, 2022
Caution With Spirulina Supplements: ConsumerLab Tests Reveal Lead Contamination, Quality Concerns
White Plains, New York, February 9, 2022 — Problems were discovered in three out of four spirulina supplements recently tested by ConsumerLab. One product was found to be contaminated with lead, and two other products, which were tablets, failed to disintegrate within the expected time.
CL Answer
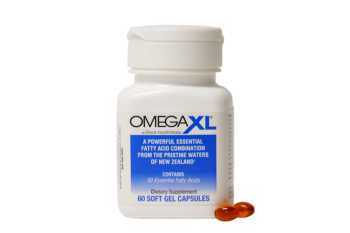
OmegaXL Review: Does It Really Work for Joint and Muscle Pain?
Read our in-depth review of OmegaXL to see if it really helps with joint and muscle pain.
CL Answer
Is it safe to drink coffee regularly? Does drinking coffee increase or decrease the risk of cancer?
Learn more about coffee, its safety, if it's good for you, and if it impacts your risk of cancer.

CL Answer
What is Skinny Fiber and does it really work? I see that glucomannan is a key ingredient. What is that?
Learn more about Skinny Fiber, including its key ingredient glucomannan, evidence from clinical studies, dosage info, and safety.

CL Answer
How much caffeine is really in dark chocolate bars?
Just how much caffeine is really in chocolate bars? Get our test results on the caffeine content in dark chocolate and cocoa powder.

Recalls & Warnings
January 20, 2021
Seller of Omega-3 Warned for Making Claims to Treat Focus, Mood
On December 9, 2020, the FDA issued a warning letter to Bodyhealth.com, LLC following a review of the company's website, which found statements made about the company's products Healthy-Thin Energize, Body Detox (Oral Spray), and Omega 3 Health to be drug claims.
Recalls & Warnings
January 12, 2021
Seller of Cholesterol, Weight Loss Products, and More Warned for Claims
On November 17, 2020, the FDA issued a warning letter to Bonagens following a review of the company's website, which found statements made about the company's products Anti-Aging Supports, Cholesterol & Diabetes Control, Gout Control, Hypertension Control, Liver Supports, Lungs ...
Clinical Update
9/23/2022
Side Effects of Dry Mouth Treatments
Be aware that many products that stimulate saliva production in people with dry mouth can cause gastrointestinal side effects. Learn more in the Salivary Stimulants section of our article dental products.
Clinical Update
6/14/2024
Is Xylitol in Toothpaste Unsafe?
Use of Xylitol was recently linked with increased risk of heart attacks, stroke, and death, but does xylitol in toothpaste or salivary stimulants for dry mouth pose a concern? Find out in our article about toothpastes and other dental products, which inlcudes our Top Picks.
Also, find out if intake of regular sugar has been linked with heart-related concerns.
Clinical Update
9/24/2024
Toothpaste for Dry Mouth
What should you look for in a toothpaste for dry mouth? Find out and see our Top Pick toothpaste for dry mouth in our article about toothpaste and other dental products. We discuss products from Sensodyne, Biotene, and Closus, and other brands.
Also see our recommendations regarding salivary stimulants, oral moisturizers, and lifestyle modifications for dry mouth.
Clinical Update
6/28/2017
Some Concern About Theobromine in Dark Chocolate
ConsumerLab's tests of dark chocolates show them to contain about as much theobromine (a caffeine-related stimulant) as heart-healthy flavanols. So is all this theobromine good or bad? A recent study suggests that it may be an issue for people who need to control their blood sugar, while, on the positive side, it lowers LDL "bad" cholesterol. Get the details in the "Concerns and Cautions" section of the Cocoa Powders, Dark Chocolate, Extracts, Nibs, & Supplements Review, which includes comparisons of the amounts of theobromine in popular products, as well as amounts of flavanols, caffeine, and contaminants.
CL Answer
Does Alpha Brain really improve memory, focus and cognition?
Find out if Alpha Brain really improves memory, focus and cognition. See the clinical evidence and get details about Alpha Brain ingredients, as well as potential side effects and drug interactions. ConsumerLab.com's answer explains.

CL Answer
What is Protandim and does it really work?
What is Protandim and does it really work? Can Protandim reduce oxidative stress? ConsumerLab's answers discusses Protandim's effectiveness, reviews and studies.

CL Answer
Does Provitalize work for weight loss and is it safe?
Find out if taking Provitalize, a probiotic supplement that also contains turmeric extract, moringa leaf extract, curry leaf extract and piperine (BioPerine), relieves joint pain, decreases abdominal fat, or helps with weight loss.

CL Answer
When taking a water-soluble version of CoQ10, do I still need to take it with food?
Find out how best to take CoQ10 supplements, with water or food.

CL Answer
Does taking a laxative interfere with the absorption of vitamins or minerals?
Information on laxative interactions with other medications and supplements such as Maalox, antibiotics, and statins and diabetes medications.

News Release
January 31, 2019
Contamination Still an Issue in Some Greens and Whole Food Products, ConsumerLab Tests Reveal
White Plains, New York, January 31, 2019 — Powders and capsules containing ingredients such as wheat grass, spirulina, chlorella, and fruits and vegetables are a popular way to get vitamins, minerals and other plant-based nutrients, but past tests by ConsumerLab have found that these products ...
Clinical Update
1/14/2017
Enzymes for Muscle Soreness
A blend of enzymes reduced delayed-onset muscle soreness after exercise in healthy men. For details, see the "What They Do" section of the Digestive Enzyme Supplements Review >>
Clinical Update
10/28/2024
Triphala for Constipation?
Can Triphala, an Ayurvedic blend of fruits from three plants, help relieve chronic constipation and is it safe? Find out in our article: Which supplements and foods help relieve constipation and which can cause constipation?
Clinical Update
6/17/2022
Probiotic for Depression?
Did a popular probiotic blend help people with severe depression? See what a recent study found in the Anxiety and Depression section of our Probiotics Supplements Review.
Also see our answer to the question: What are the best supplements for depression and anxiety?
News Release
August 08, 2016
ConsumerLab Finds Lead, Cadmium and Arsenic Contamination in Greens and Whole Foods Supplements
White Plains, New York, August 9, 2016 — Powders, pills and drinks made from ingredients like wheat grass, alfalfa, kelp, spirulina, chlorella, leafy vegetables and fruits are a popular choice for people looking to increase their intake of vitamins, minerals and other plant-based nutrients.
Clinical Update
3/31/2023
Olive Oil Review Expanded: Many Do Not Make the Grade
We tested three new olive oils based on reader requests, and the results are in! Our new tests included two olive oils that claim to be high in polyphenols, and the results were surprising:
-- One olive oil was rated so poorly by our professional taster it was considered to be "lampante" -- i.e., not fit for human consumption.
-- The other high-polyphenol oil delivered a good amount of polyphenols, rated well for taste, and is now our new Top Pick for a very high-polyphenol olive oil.
We also tested California Olive Ranch Global Blend but were somewhat disappointed.
See the results now for 13 popular olive oils in our updated Extra Virgin Olive Oil Review.
News Release
November 06, 2015
Some Surprising Results from Tests of 43 Probiotic Supplements and Kefir Drinks
White Plains, New York, November 6, 2015 — How many beneficial organisms are in probiotic supplements and kefir drinks? Are any contaminated with pathogens, such as E. coli, and do those labeled as "gluten free" or "99% lactose-free" live up to their claims? To find out, ConsumerLab.
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
December 26, 2023
Total Body Nutrition, TBN Labs, and Loud Muscle Science Agree to Stop Selling Adulterated and Misbranded Dietary Supplements
On December 13, 2023, Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science LLC, as well as the companies’ owner, Mohammed Islam, agreed to stop manufacturing and distributing adulterated and misbranded dietary supplements and to comply with dietary supplement current good ...
News Release
July 07, 2014
Which Weight Loss Supplements Are Best? ConsumerLab.com Reviews the Evidence and Tests the Quality of Popular Products
White Plains, New York, July 7, 2014 — Dozens of supplement ingredients have been touted for weight loss, but which have the strongest evidence showing they work and, among those, which products are highest in quality? To answer these questions, ConsumerLab.
Recalls & Warnings
January 06, 2025
ZOE Prebiotic Blend Recalled Due to Metal Pieces, Stones
On December 4, 2024, Zoe US, Inc. recalled 142 units of ZOE Daily 30+ Prebiotic blend because it may contain foreign objects such as small metal pieces and/or stones.
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
Recalls & Warnings
March 26, 2021
Nine Banned Stimulants Found in Workout, Weight Loss Supplements
Recent analysis of 17 brands of sports/energy and weight loss supplements sold in the U.S. found nine prohibited stimulants formulated into eight different combinations, and none of these combinations have been studied in people.
Recalls & Warnings
February 05, 2025
Muscle Tech Alpha Test Recalled
On December 18, 2024, Iovate Health Sciences USA Inc. recalled 10 lots (163,248 units) of Muscle Tech Alpha Test capsules because they contain cathine, a controlled substance that can cause serious adverse effects.
News Release
January 15, 2014
Problems Found with the Quality and Labeling of Some "Muscle Enhancement" Supplements -- Review of Creatine and Branched-chain Amino Acid Supplements Published by ConsumerLab.com
White Plains, New York, January 15, 2014 — Athletes often turn to supplements such as creatine and branched-chain amino acids (BCAAs) to enhance muscle strength and recovery. These supplements may also benefit people with muscular diseases and those recovering from knee surgery.
News Release
March 02, 2010
ConsumerLab.com finds carcinogenic form of chromium in supplements, including those for weight loss -- Reviews published of supplements containing chromium, green tea, 7-keto DHEA and stimulant formulas
White Plains, New York — March 2, 2010 — A carcinogenic form of chromium, hexavalent chromium, is present is some dietary supplements, according to new tests by ConsumerLab.com.
Recalls & Warnings
December 09, 2021
Florida Man Convicted for Distributing Steroids Labeled as Dietary Supplements
On December 9, 2021, 37-year-old Florida resident James Boccuzzi was convicted of one count of conspiracy to defraud the U.S. Food and Drug Administration (FDA) and one count of conspiracy to distribute controlled substances.
News Release
August 25, 2009
ConsumerLab.com tests quality of acai berry supplements and beverages -- No contamination found but health benefits remain unproven. Caution urged with laxative formulas and billing schemes.
White Plains, New York — Tuesday, August 25, 2009 — Acai berries have antioxidant properties and are a staple of the traditional Brazilian diet. In recent years, supplements and drinks made from acai have become popular in the U.S.
News Release
November 15, 2005
Testing by ConsumerLab.com identifies many problems with popular supplements for weight loss, slimming, and blood sugar control
Westchester, NY — November 15, 2005 — In a series of reports released today, ConsumerLab.com revealed test results for supplements used for weight loss, slimming, and blood sugar control. Among the twenty-three products that ConsumerLab.
Recalls & Warnings
October 09, 2023
Organifi Settles Charges of Unsupported Claims
Organifi, LLC has agreed to pay $150,000 in civil penalties and $50,000 in investigative costs as restitution to settle charges that the company made unsupported claims that its Gold, Pure, Green Juice, and Red Juice products could balance hormone and cortisol levels, regulate the ...
News Release
April 22, 2004
ConsumerLab.com shares insights from 2 years of testing supplements for athletic banned substances — Athletes and manufacturers urged to learn how to avoid problems
WHITE PLAINS, NY — April 22, 2004 — Two years after initiating the first program to test dietary supplements for substances banned from athletic competition, ConsumerLab.com today released a short advisory summarizing problems uncovered to-date from its testing.
News Release
January 09, 2004
USANA products pass ConsumerLab.com screening for substances banned from Olympics
WHITE PLAINS, NY — January 9, 2004 — ConsumerLab.com announced today that six additional products have passed its Athletic Banned Substances Screening Program. ConsumerLab.com tested the products at the request of USANA, a supplement manufacturer. ConsumerLab.
News Release
August 20, 2002
ConsumerLab.com announces screening of supplements for banned substances for the United States Olympic Committee — First products to pass now listed on ConsumerLab.com web site
WHITE PLAINS, NY — August 20, 2002 — The United States Olympic Committee (USOC) and ConsumerLab.com (CL) announced today that nine products have passed ConsumerLab.com's recently launched Athletic Banned Substances Screening Program. ConsumerLab.
News Release
February 08, 2002
Athletic Banned Substances Screening and Certification Program announced by ConsumerLab.com — Tester of supplements to screen for substances banned in Olympics
WHITE PLAINS, NY — February 8, 2002 — ConsumerLab.
News Release
May 07, 2001
Over forty percent of Echinacea products fail ConsumerLab.com review; Inadequate labeling and missing ingredients found common for popular herbal cold remedy
WHITE PLAINS, NY — May 7, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of echinacea supplements.
Recalls & Warnings
January 24, 2020
Fat-Burning, Energy Supplement Linked to Heart Trouble
A 33-year-old woman in Australia developed cardiac ischemia (a lack of blood flow to the heart) after taking the "fat burning" supplement Alpha Lean-7, according to a recent report in the Journal of Sports Sciences.
Recalls & Warnings
December 12, 2022
FDA Warns Saffron USA for Promoting Teas to Treat Insomnia, Osteoporosis & Cancer
On September 23, 2022, the FDA issued a warning letter to Saffron USA LLC following inspection of the company’s website which found statements about its Allergy Blend, Chamomile Tea Petals, Diabetic Support Blend, Orange Blast Tea, and Saffron Loose Tea products to be drug ...
Recalls & Warnings
July 09, 2010
FDA Issues Warning on Herbal Weight Loss Supplement
On July 8, 2010, the U.S. Food and Drug Administration (FDA) warned that Que She, marketed as an herbal weight loss supplement, contains active pharmaceutical ingredients not listed on the product label that could harm consumers, especially those with cardiovascular conditions.
Recalls & Warnings
September 09, 2018
Higenamine -- A Potentially Dangerous Stimulant -- Found in Some Supplements
A recent study found concerningly high doses of the stimulant higenamine in some weight loss, energy and sports supplements sold in the U.S. Higenamine is a naturally-occurring stimulant which is permitted to be sold as a dietary supplement ingredient in the U.S.
Recalls & Warnings
November 24, 2020
Recalled Vitamin D Contains Potentially Dangerous Ingredient
On November 22, 2020, Fusion Health and Vitality LLC recalled all 2020 lots of CORE Essential Nutrients and Immune Boost Sublingual Vitamin D3 because they are adulterated. CORE Essential Nutrients contains the unapproved food additive hordenine HCl.
Recalls & Warnings
June 16, 2020
NutraCap Labs Warned for Unsafe Ingredients and Manufacturing Violations
On May 21, 2020, the FDA issued a warning letter to NutraCap Labs LLC, which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
December 02, 2020
Seller of Vitamin C, Elderberry, Silver, and More Warned for COVID-19 Claims
On November 10, 2020, the FDA issued a warning letter to Sage Woman Herbs, Ltd.
Recalls & Warnings
November 10, 2020
Seller of Cholesterol-Lowering Supplements Warned for Drug Claims
On October 29, 2020, the FDA issued a warning letter to Natural Sprout Co.
Recalls & Warnings
August 11, 2008
Stroke Attributed to Xenadrine Ephedra-Free Supplement
A case report in the journal Military Medicine (July 2008) attributes the occurance of vasospasm and stroke in a 36 year-old women to use of Xenadrine-EFX, a supplement used for weight loss.
Recalls & Warnings
April 06, 2016
FDA Warns of Stimulant Methylsynephrine In Supplements
On March 31, 2016, the FDA issued warning letters to seven companies selling products containing methylsynephrine (also called p-hydroxyephedrine or Oxilofrine), a stimulant drug which is not permitted in dietary supplements in the U.S.
Recalls & Warnings
April 16, 2019
DMHA and Phenibut Are Not Permitted in Dietary Supplements, Warns FDA
On April 16, 2019, the FDA announced it has issued 11 warning letters to companies whose dietary supplement products contain the drugs DMHA or phenibut, and therefore are in violation of the law.
Recalls & Warnings
November 24, 2018
Dangerous Drug In Supplements for Pain and Anxiety
On November 20, 2018, the FDA announced it has issued warning letters to two companies for the illegal marketing of products labeled as dietary supplements that contain the opioid-like drug tianeptine.
Recalls & Warnings
April 08, 2016
Large Doses of Stimulant Methylsynephrine Found In Weight Loss Supplements
Amounts of methylsynephrine found in some supplements exceed prescription dosages, according to new study published in Drug Testing and Analysis.
Recalls & Warnings
September 30, 2015
Weight and Energy Supplement Contains Synthetic "Amphetamine"
On September 21, 2015, the FDA issued a warning letter to TruVision Health LLC. because the company's supplement tru Weight & Energy lists a synthetic, amphetamine-like compound, AMP (also called 1,3-dimethylbutylamine or DMBA) on product labels.
Recalls & Warnings
August 22, 2015
Synthetic Amphetamine, Less Protein and More Sugar Than Claimed Found in Workout Supplements
On August 7, 2015, the FDA issued a warning letter to New Dawn Nutrition, Inc.
Recalls & Warnings
April 29, 2015
FDA Targets Weight Loss and Workout Supplements Listing Synthetic Stimulant DMBA
On April 24, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called 1,3-dimethylbutylamine (DMBA) on product labels.
Recalls & Warnings
November 25, 2016
DMAA Product Recalled
On November 23, 2016, NutriVitaShop (a dba of Naturecom Inc.) issued a recall of its DMAA net weight 500g because it may contain DMAA.
Recalls & Warnings
October 09, 2014
New Synthetic Stimulant Found in Supplements
A synthetic stimulant never before studied in humans, 1,3-dimethylbutylamine (DMBA), was found in a dozen supplements sold by U.S. distributors in dosages from 13 to 20 mg per serving (Cohen, Drug Test Analys 2014).
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
July 20, 2017
Jail Time for Seller of Spiked Supplements
On July 18, 2017, Derek Vest, the former president of Gentech Pharmaceutical located in Fort Meyers, Florida, was sentenced to 18 months in prison for introducing misbranded food into interstate commerce.
Recalls & Warnings
April 29, 2015
Teen Use of Weight Loss and Workout Supplements Labeled "For Adult Use Only" Concerning
Three new studies have raised concerns that teenagers are able to purchase "body-shaping" supplements, such as creatine, testosterone boosters, and "fat-burning" products from health food stores despite the fact that many of these supplements are labeled "for adult use only.
Recalls & Warnings
February 16, 2016
Work Out and Weight Supplements Contain Synthetic Amphetamine-Like Compound
On February 3, 2016, the FDA issued a warning letter to ATS Labs, LLC because labels for the company's work out and weight loss supplements CFI, Weapon-X Pre-Workout Extreme, and Lady Lean list a synthetic, amphetamine-like compound, 4-amino-2-methylpentane citrate (also called ...
Recalls & Warnings
October 08, 2010
FDA Warns of Stimulant in Slimming Capsules
On October 8, 2010, the U.S. FDA advised consumers who have Slimming Beauty Bitter Orange Slimming Capsules not to use the product. FDA warns that Slimming Beauty Bitter Orange Slimming Capsules contain the active pharmaceutical ingredient sibutramine, a prescription-only drug which is a stimulant.
Recalls & Warnings
July 11, 2011
Use of "Sports Drinks" and "Energy Drinks" Discouraged by Pediatric Group
A report from the American Academy of Pediatrics outlines how sports and energy drinks are being misused and provides guidance to decrease or eliminate consumption by children and adolescents.
Recalls & Warnings
April 05, 2016
Muscle Enhancement Supplement Recalled
On April 5, 2016, Invisiblu International LLC issued a recall of one lot of the muscle enhancement supplement Continuum Labs LGD-Xtreme because it contains LGD-4033 Ligandrol. The risks of this ingredient are not known.
Recalls & Warnings
December 23, 2016
Twelve Sexual Enhancement Supplements Found to Contain Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the following twelve sexual enhancement supplements because they were found to contain undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
August 07, 2011
Performance Enhancing Ingredient, DMAA, Not Really from Geraniums, Putting Its Use in Supplements in Doubt
On July 29, 2011, the American Herbal Products Association (AHPA) announced a new requirement that products containing the sports performance enhancing compound DMAA (also known as 1,3-dimethlypentlyamine, 1,3 dimethylamylamine, methylhexanamine, or MHA), not be labeled as geranium oil or any part ...
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
December 13, 2013
Maker of Menopause Blend and Other Supplement Ingredients Warned by FDA of Violations
On November 21, 2013, the FDA issued a warning letter to Raw Deal, Inc.
Recalls & Warnings
January 21, 2015
Smoothie Blends Recalled Due to Potential Listeria Contamination
On January 18, 2015, Inventure Foods, Inc. issued a recall of RADER FARMS Fresh Start Smoothie Blend, Fresh Start Sunrise Refresh Fusion, and Fresh Start Daily Power Fusion, because they have the potential to be contaminated with Listeria monocytogenes.
Recalls & Warnings
June 13, 2012
Lack of Quality Control and Unapproved Claims by Supplement Seller
On May 14, 2012, the U.S. FDA sent a Warning Letter to Caribe Natural, LLC, distributor of GERMA brand dietary supplements, regarding violation of various FDA requirements.
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
Recalls & Warnings
February 06, 2013
Ingredient Supplier Warned For Meal Replacement Product Misbranding and Adulteration
On October 4, 2012, the FDA issued a warning letter to dietary supplement ingredient supplier Raw Deal, Inc.
Recalls & Warnings
November 12, 2017
Weight Loss and Cleanse Supplements Contain Adulterated and Improperly Labeled Ingredients, FDA Warns
On November 2, 2017, the FDA issued a warning letter to Create-A-Pack Foods, Inc.
Recalls & Warnings
January 31, 2015
Protein Powder Recalled Due to Food Poisoning Risk
On January 30, 2015, Aloha, Inc. issued a voluntary recall of all packages of Premium Protein powder blends in chocolate and vanilla because they have the potential to be contaminated with Staphylococcus enterotoxin .
Recalls & Warnings
January 24, 2013
Food for Health Warned for Manufacturing Violations and Drug Claims on Supplements
On October 5, 2012, the FDA issued a warning letter to Food for Health International, LLC following a facility inspection which found the company's products, including Activz brand Vitamin D, Potassium Iodine, Organic Vitamin C, Whole 9 (a fruit and vegetable meal replacement shake) Control and VMA ...
Recalls & Warnings
July 11, 2017
Protein Powder Recalled Due to Allergen Risk
On July 7, 2017, Biohealth Nutrition recalled its Precision Blend Cookies & Cream because it contains undeclared wheat.
Recalls & Warnings
March 04, 2003
U.S. Warns of Ephedra Risks and Proposes Warning Label for Supplements
On February 28, the Department of Health and Human Services (HHS) announced a series of actions designed to protect Americans from potentially serious risks of dietary supplement products containing ephedra.
Recalls & Warnings
January 24, 2013
"Thermo Stimulating" Supplement Recalled -- Contained DMAA
On January 23, 2013, Health Canada advised consumers not to use certain batches of Muscletech Hydroxystim capsules which were found to contain DMAA (1,3-dimethylamylamine) and have been recalled by their distributor.
Recalls & Warnings
February 07, 2013
Growth Factor Supplement For "Size, Strength and Stamina" Recalled Due To Potential Contamination
On January 2, 2013, EonNutra, LLC issued a voluntary recall of specific batches of Soto Supplements Growth Factor Complex 200 GFC 200 (2 fl oz.) liquid dietary supplement due to potential bacterial contamination.
Recalls & Warnings
November 11, 2015
Consuming a Single Energy Drink May Increase Cardiovascular Risk
A recently published study found that consumption of a single caffeinated energy drink significantly increased blood pressure and noradrenaline levels in healthy young adults. The researchers noted these changes could potentially increase the risk of cardiovascular events.
Recalls & Warnings
January 23, 2014
Many Thyroid Supplements Contain Thyroid Hormones -- Risk of Thyrotoxicosis
A recent study found that 9 out of 10 supplements marketed for "thyroid support" and purchased at retail stores or on the Internet contained actual thyroid hormones thyroxine (T4) and/or triiodothyronine (T3), posing a risk to consumers.
Recalls & Warnings
July 19, 2014
Powdered Caffeine Can Be Lethal, FDA Warns
On July 18, 2014, the FDA warned consumers to avoid powdered pure caffeine sold in bulk bags over the internet.
Recalls & Warnings
November 20, 2013
$2 Million In Weight Loss and "Fat-Burning" Supplements Seized
On November 12, 2013, U.S. Marshals seized more than $2 million worth of supplements from Georgia-based Hi-Tech Pharmaceuticals, Inc.
Recalls & Warnings
October 16, 2013
Methamphetamine-Like Compound Found In Popular Workout Supplement
A study published on October 14, 2013 (Cohen, Drug Test Analysis 2013) reports that the popular pre-workout energy supplement Craze (Driven Sports) has been found to contain the banned methamphetamine — like compound, N, á-diethylphenylethylamine.
Recalls & Warnings
September 05, 2015
Powdered Caffeine Risky, FDA Warns
On August 27, 2015, the FDA issued a warning letter to five companies selling caffeine powder, warning that the powder "presents a significant or unreasonable risk of illness or injury under the conditions of use recommended or suggested in the labeling."
Recalls & Warnings
May 26, 2017
"Allergy" Supplement Containing Ephedra Recalled
On February 7, 2017, MusclMasster, LLC (DBA The Green Herb) of Wheat Ridge, CO issued a recall of all bottles of Al-Er-G Capsules , a product promoted to help allergies, because they contain ephedra.
Recalls & Warnings
February 09, 2017
Herbal Supplement Found to Contain Ephedra Alkaloids Recalled
On February 7, 2017, Kingsway Trading Inc. issued a recall of Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains ephedra alkaloids.
Recalls & Warnings
December 29, 2016
Sexual Enhancement Supplement Contains Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Power Male Sexual Stimulant because it was found to contain sildenafil. This supplement was identified during an examination of international mail shipments.
Recalls & Warnings
May 30, 2017
Seller of Vitamin C, Calcium, Mushroom Supplements and More Warned for Manufacturing Violations
On May 12, 2017 the FDA issued a warning letter to VitaPurity Corporatio.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
December 17, 2004
FDA Seizes Ginseng Because of Potentially Risky Pesticide Residues
On December 16, 2004, the Food and Drug Administration (FDA) announced that the U.S. District Court for the District of New Jersey issued a warrant for the seizure of imported ginseng held for sale at FCC Products, Inc., located in Livingston, N.J. The U.S.
Recalls & Warnings
July 23, 2013
Weight Loss Supplements Containing Undeclared Drugs Recalled
On July 19, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss supplements Esbelin siloutte te and Esbelin siloutte Herbal Blend with L-Carnitine because they were found to contain undeclared sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
July 12, 2013
Herbal Supplement Company Warned For Drug Claims, Manufacturing Violations
On June 18, 2013, the FDA issued a warning letter to Herbs of Light, Inc.
Recalls & Warnings
March 21, 2013
FDA Warns Consumers: Three Sexual Enhancement Supplements Found To Contain Drugs
On March 21, 2013, the FDA warned consumers not to purchase or use three sexual enhancement dietary supplements, Rock-It Man, Libido Sexual Enhancer, and Stiff Days, because they were found to contain sildenafil or sildenafil analogues.
Recalls & Warnings
February 13, 2013
FDA Warns Maker of Weight Loss and Whey Protein Supplements For Manufacturing Violations and Misbranding
January 25, 2013, the FDA issued a warning letter to dietary supplement manufacturer SciLabs Nutraceuticals, following a facility inspection which found the company's dietary supplements and supplement ingredients, including N-Large 2, Flo-Gard AB, Nitrogen Glutamine capsules, Nutritech nutritional ...
Recalls & Warnings
March 03, 2006
FDA Requests Seizure of More Supplements Containing Ephedrine Alkaloids
On February 24, 2006, the U.S. Food and Drug Administration (FDA) announced that the U.S.
Recalls & Warnings
November 29, 2012
Sensa Settles Second False Advertising Lawsuit
On November 27, 2012, Sensa Products LLC, maker of the Sensa Weight Loss System, announced it agreed to settle a false advertising lawsuit filed by the Nutritional Supplemental Task Force in California, without an admission of guilt.
Recalls & Warnings
April 29, 2012
FDA Says DMAA Can't Be Sold as a Supplement -- Warns Sellers
On April 27, 2012, the U.S.
Recalls & Warnings
July 26, 2013
Safety of Certain Weight Loss and Bodybuilding Supplements Called Into Question
On July 25, 2013, USA Today reported that several supplements, including weight loss, body building and sports nutrition supplements, have come under scrutiny after being found to contain illegal ingredients, or were associated with failed drug tests and adverse events.
Recalls & Warnings
March 01, 2013
Manufacturer of Joint, Weight Loss, Muscle Supplements and More Warned for Adulteration, Misbranding and Drug Claims
On February 4, 2013, the FDA issued a warning letter to DC Nutrition, Inc.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
July 27, 2012
Prescription and Street Drug Alternatives Found in Male Enhancement, Mood, and Sleep Supplements
On July 10, 2012, the FDA issued a warning letter to Evol Nutrition Associates, Inc. subsequent to a 2011 inspection which found a number of the company's products contained prescription and investigational drugs and were misbranded.
Recalls & Warnings
January 11, 2013
Consumers May Be Eligible For Cold, Allergy and Weight Loss Supplement Refunds
On January 7, 2013, the Federal Trade Commission (FTC) announced that consumers who purchased the Iovate Health dietary supplements Accelis, nanoSLIM, Cold MD, Germ MD, or Allergy MD between January 2006 and July 2010 may qualify for a refund.
Recalls & Warnings
March 04, 2008
Airborne Settles Lawsuit Over False Advertising of its "Miracle Cold Buster"
On March 4, 3008, the Center for Science in the Public Interest (CSPI) announced that the makers of Airborne -- a multivitamin and herbal supplement whose labels and ads falsely claimed that the product cures and prevents colds -- will refund money to consumers who bought the product, as part of a ...
Recalls & Warnings
September 10, 2012
Maker of Concussion Recovery Supplement Warned For Making Drug Claims
On August 28, 2012, the FDA issued a warning letter to Trinity Sports Group, Inc. after a review of the company's websites found statements about Neuro Impact Concussion Response Formula constituted drug claims not permitted on supplements.
Recalls & Warnings
April 19, 2013
Recall: Protein Powder May Have Been Contaminated During Packaging
On March 14, 2013, F.H.G Corporation, dba Integrity Nutraceuticals, issued a voluntary recall of MusclePharm Cookies 'N" Cream Combat Powder and Banana Cream Combat Powder protein powders because they may be contaminated with extraneous materials due to problems on the packaging line.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
April 03, 2014
Maker of Green Coffee Bean Extract and Weight Loss Supplement Warned for Manufacturing Violations
On March 13, 2014, the FDA issued a warning letter to Libi Labs, Inc.
Recalls & Warnings
March 19, 2014
Maker of Herbal Capsules and Extracts Warned for Manufacturing Violations
On September 19, 2013, the FDA issued a warning letter to Herbalist and Alchemist, Inc.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
Recalls & Warnings
November 21, 2015
FTC Charges Maker of Supplement for Opiate Addiction with Deceptive Claims
On November 16, 2015, the FTC filed a lawsuit against Sunrise Nutraceuticals, LLC, charging that the company made deceptive claims its product, Elimidrol, can alleviate opiate withdrawal symptoms and increase a user's likelihood of overcoming opiate addiction.
Recalls & Warnings
January 17, 2006
FDA Warns Consumers about Brazilian Diet Pills Found to Contain Active Drug Ingredients
On January 13,2006, the U.S. Food and Drug Administration (FDA) warned consumers not to use two unapproved drug products that are being marketed as dietary supplements for weight loss.
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...






