Reviews and Information for Matcha Green Tea
Green Tea Supplements and Drinks
If you want to find the best green tea high in antioxidants such as EGCG, you'll want to see our laboratory findings. We discovered a shocking range of EGCG, from 8 mg to more than 450 mg per serving! See results for popular brands of green tea as tea bags, loose leaf tea, bottled tea, ceremonial matcha powder, and green tea extract supplements. Also see how much caffeine we found, compare prices, and learn what conditions green tea is good for and about potential side effects.
Green Tea Supplements and Drinks ReviewSearch term may appear only in full report available to members. Join now for full access.
CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

News Release
February 15, 2024
Unveiling the Truth About Green Tea’s EGCG Content
White Plains, NY, February 15, 2024 — ConsumerLab.com, a trusted name in health and wellness, sheds light on the EGCG (epigallocatechin gallate) content in green tea.
News Release
February 24, 2023
Probiotics Rise in Popularity as Vitamin C, Melatonin, and Others Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 25, 2023 —A recent survey of 8,600 people who regularly use dietary supplements shows that probiotics (+3.04 percentage points), quercetin (+2.3 pts), and vitamin K (+1.
Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Clinical Update
2/27/2024
Matcha Green Tea
We recently announced our Top Pick for "ceremonial" matcha in our Green Tea Review. A CL member asked if we’ve tested "culinary matcha." Although we did not this year, we did in 2021 and we have added those results to the current report.
Clinical Update
9/12/2024
Matcha for Sleep?
Does taking green tea matcha powder before bedtime improve sleep? Find out what a recent study showed in the Sleep section of our Green Tea Supplements Review, which includes our Top Picks among matcha powders and other green tea products.
Clinical Update
5/19/2025
Lead in Matcha Green Tea
An independent group recently tested 9 matcha green tea powders and reported that levels of lead and other heavy metals far exceeded "Action Levels." Get our take on this and how the results to compare our own in our Green Tea Review, which includes our Top Picks for matcha and other green teas.
Product Review
Lion's Mane and Chaga Supplements Review
Read Labels Carefully -- Many Can Mislead

Clinical Update
2/27/2018
Matcha Green Tea for Memory & Cognition?
Can drinking matcha green tea enhance memory or have other cognitive benefits? Find out what a recent study showed in the Memory and Cognition section of the Green Tea Review.
Clinical Update
6/30/2020
Getting the Most from Green Tea
Our Green Tea Review shows that matcha green tea provides higher amounts of healthful catechins than regular green tea. This week, we added information about why this is as well as the results of a new study showing which type of matcha provides the highest levels of polyphenols (i.e., catechins). For details see the What to Consider When Buying section of the review. Also see our Top Picks among green tea products.
CL Answer
How significant are the risks to drinking tea from China from heavy metals?
Find out which types of tea from China pose the greatest risk of toxic heavy metal contamination, including green tea.

News Release
May 10, 2018
Big Differences Found Among Green Teas -- ConsumerLab Tests Identify Best Green Tea in Bags, Matcha, Supplements, and Bottles --
White Plains, New York, May 10, 2018 — The benefits of green tea use are associated with the presence of natural polyphenols known as catechins, such as EGCG.
CL Answer
Which foods and supplements contain lead, cadmium, or arsenic?
Discover which foods and dietary supplements contain lead and other metals, the limits, who’s testing, and how to stay safe.

Product Review
Olive Leaf Extract Supplements Review
Learn What Olive Leaf Does and the Best Brands

Product Review
Electrolytes & Sports Drinks Review
See Which Sports Drinks, Powders, and Pills Deliver the Right Electrolytes
Product Review
Lavender and Tea Tree Essential Oils Review
Oils Tested for Authenticity and Purity

Clinical Update
3/11/2022
Green Tea and Colon Polyps
Can taking green tea extract reduce the recurrence colon polyps? See what a recent study found in the What It Does section of our Green Tea Review. Also see our Top Picks among green tea extracts, tea bags, loose tea, and matcha powders.
See our answer to the question: Which supplements help reduce the risk of colorectal cancer?
Clinical Update
3/27/2018
Green Tea and Ovarian Cancer
Drinking green tea was associated with a reduced risk of developing ovarian cancer in a recent analysis. Get the details about this and green tea's relationship to other types of cancer in the What It Does section of the Green Tea Review. (Also see our test results for various types of green tea, including matcha, bagged tea, loose tea, and supplements.)
Clinical Update
10/16/2018
A Caution With Green Tea
Although consumption of green tea is generally associated with a reduced risk of cancers, drinking it a certain way may increase the risk of certain cancers. Get the details in the Cancer Prevention section of the Green Tea Review. (Also see our Top Picks among green tea in bags, bottles, supplements, and matcha.)
Clinical Update
10/27/2018
Cleaner Green Tea
A recent study found that it is possible to modestly reduce pesticides in green tea by following a certain procedure before brewing. For details, see the How to Take section of the Green Tea Review. (Also see our Top Picks among green teas, matcha powders, and supplements.)
Clinical Update
11/25/2017
How Hot Should Green Tea Be?
Drinking green tea may reduce the risk of certain cancers, but its effect on gastric (stomach) cancer may depend on the temperature at which you drink it, according to a new study. Find out what the best temperature seems to be in the "What It Does" section of the Green Tea Review >> (Also see how brands of green tea, including matcha, stack up in our tests.)
Clinical Update
3/10/2020
Staying Safe With Green Tea
If you use a green tea extract (alone or as part of a supplement formula), it is important to take it with food according to several reports, as explained in the Concerns and Cautions section of the Green Tea Review. Also see our Top Picks for green tea in bags, loose, as matcha powder, and in other forms.
Clinical Update
8/27/2020
Is Expired Green Tea Safe?
We were asked if it is safe to use green tea past its "best by" date and if its flavor or health benefits would be diminished. See our answer in the How to Use section of our Green Tea Review. Also see our Top Picks for green tea — including tea bags, loose tea, matcha, and green tea supplements.
Clinical Update
5/29/2025
"Forever Chemicals" In Green Tea?
Testing by a separate research group identified detectable levels of PFAS ("forever chemicals") in one-third of popular brands of green tea, according to a recent report. Get the details, and see our take on the evidence, in the Concerns and Cautions section of our Green Tea Supplements Review, which includes our Top Picks for green tea bags, matcha powder, and green tea supplements.
Clinical Update
6/16/2025
Bigelow Tea False Claim
A jury recently awarded consumers of certain Bigelow teas $2.3 million after finding a "Manufactured in the USA" claim to be false. Get the details in our Green Tea Review, which includes our Top Picks among popular green tea bags, loose tea, matcha tea, and bottled tea.
Clinical Update
3/31/2025
Plastic in Tetley Tea Bags?
Find out if Tetley tea bags contain plastic in the Concerns with Plastic Tea Bags section of our Green Tea Review, which includes information about plastics in tea bags from Bigelow, Numi, Republic of Tea, Tazo, Trader Joe’s, and Twinings.
Also see our Top Picks for green tea (including tea bags, loose leaf tea, bottled teas, matcha, and supplements).
CL Answer
Does coffee have brain benefits?
People commonly drink coffee to increase alertness, counteract fatigue, and boost cognition, but does it really improve memory and cognitive function? Find out, and learn about risks linked with drinking too much coffee.

Product Review
Weight Loss Supplements Review (7-Keto DHEA, Forskolin and Stimulant Blend Supplements)
Choose the Best Weight Loss Supplement. Be Careful With Weight Loss Supplements — Few Pass Quality Testing and Safety Review.

Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Product Review
Amla Supplements Review
Only 3 of 8 Amla Powders and Pills Pass CL's Tests

Product Review
L-Theanine Supplements Review
Touted for Stress Relief, Do L-Theanine Supplements Work?
CL Answer
Does Rooibos Tea Have Health Benefits and Is It Safe?
Learn about the health benefits of rooibos tea, including red and green, unfermented rooibos tea, how is differs from black and green tea, safety, and potential drug interactions with rooibos tea.

CL Answer
Does drinking coffee deplete vitamins or minerals, such as magnesium, in the body? Can adding magnesium or other minerals reduce the acidity or improve the flavor of coffee?
Coffee can reduce absorption or increase loss of certain vitamins and minerals. Find out which are affected, and what steps can be taken to reduce the effect of coffee on these nutrients.

CL Answer
Do any supplements help with gum disease or periodontitis?
Supplements for healthy gums may help with gum disease or periodontitis. Learn more about omega fatty acids, lycopene, and probiotics.

CL Answer
I take lisinopril (Zestril), an ACE inhibitor drug to lower blood pressure. Are there supplements I should avoid, or be taking, due to this drug?
When taking ACE inhibitors, find out which supplements should be avoided. Interaction info on Lisinopril or another ACE inhibitors.
CL Answer
Does Gynostemma pentaphyllum really work as an AMPK activator and help for diabetes, high cholesterol, or weight loss?
Find out if Gynostemma pentaphyllum ( jiaogulan) works as an AMPK activator and helps to lower blood sugar and treat diabetes. ConsumerLab.com's answer explains.

CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

CL Answer
Dandelion Tea & Supplements: Health Benefits and Safety
Find out about the potential health benefits of dandelion tea and supplements, as well as safety, side effects, and potential drug interactions.

CL Answer
5 Tips for Finding a Soap or Body Wash That Won't Irritate Skin
Get 5 tips for finding soap or body wash that won't cause skin irritation. See which soaps we rate best, ingredients to avoid, and phrases on labels that can mislead.

CL Answer
Which supplements should be taken with food?
Find out which supplements should be taken with food, including vitamins A, D, E, and K, fish oil, and CoQ10.

CL Answer
How does Athletic Greens’ AG1 compare to multivitamins and Greens powders? Is AG1 worth it?
ConsumerLab reviews the ingredients in AG1, its safety and possible side effects, and how AG1 compares to other products.

CL Answer
Which vitamins and supplements are good for acne, and are there any that make it worse?
Information about supplements for acne and clearer skin. Plus, find out which vitamins and supplements can make acne worse.

CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
CL Answer
Which supplements and foods reduce or increase the risk of kidney stones?
Find out which supplements, foods and beverages may increase or decrease the risk of kidney stones, including information about potassium, calcium, choline, turmeric and curcumin, vitamin C, as well as foods and beverages such as green tea, lemon juice, orange juice, cranberry juice, chocolate and almonds.

CL Answer
How much caffeine is really in dark chocolate bars?
Just how much caffeine is really in chocolate bars? Get our test results on the caffeine content in dark chocolate and cocoa powder.

CL Answer
Contamination of Dry Shampoos with Benzene — What You Need to Know
Benzene, a carcinogen, has been detected in several dry shampoo aerosol sprays. Find out which dry shampoos were found to contain benzene and how much was in each one, plus how tips for reducing your exposure to benzene.

CL Answer
Can drinking coffee weaken bones or make arthritis worse?
Learn about the effects of coffee intake on bone mineral density, risk of osteoporosis, and risk of fracture. Also, find out if drinking coffee might increase the risk of arthritis or worsen symptoms.

Clinical Update
4/13/2014
Does Green Tea Improve Memory?
This week, news sources reported that a study found green tea helps working memory. While green tea may have benefits, a closer look at this study suggests the news was exaggerated and misleading. For details about the study (and information about other effects of green tea and our tests of green tea supplements, teas, and drinks) see the update in the Green Tea Supplements, Drinks and Teas Review >>
CL Answer
Are supplements which claim increased absorption or improved bioavailability telling the truth? Is it worth paying more for these? Are there concerns?
Learn more about bioavailability enhancers, including emulsifiers and enzyme inhibitors.

Clinical Update
12/17/2019
Green Tea and Breast Cancer Risk
Is there a relationship between green tea consumption and breast cancer risk? Learn what a large recent study showed in the Cancer Prevention section of the Green Tea Review. Also see our Top Picks for green tea -- including brewable green tea (from bags and loose tea) and green tea drinks.
Clinical Update
6/03/2015
Green Tea for Weight Control?
Many studies have looked at whether green tea can help with weight management. The results have been mixed. A recently published study used a high-dose green tea extract. Although the researchers concluded it worked, a closer look suggests otherwise, and points to a potential risk. For details, as well as the results of our tests of green tea supplements, beverages, and brewable green tea, see the Green Tea Review >>
Clinical Update
9/16/2024
Green Tea & Iron Deficiency
Severe iron-deficiency anemia was recently reported after regular consumption of green tea. Get the details in our Green Tea Review, which includes our Top Picks for green tea.
Also be aware that drinking green tea may significantly affect blood levels of a common beta-blocker, as well as statins, blood-thinners, and thyroid medication. See the Concerns and Cautions section of our Green Tea Review.
Clinical Update
6/20/2014
Green Tea and Liver Toxicity
Green tea extract was listed as one of the most common dietary supplements linked to liver injury in a newly published guideline for diagnosing and treating drug-induced liver injury. Find out what one of the authors of the guideline had to say about the catechin content of some green tea supplements, and get our test results showing the amounts of catechins in green tea supplements in the Green Tea Supplements, Drinks and Brewable Teas Review >>
Clinical Update
8/09/2015
Green Tea May Inhibit Starch Absorption
A study recently found that taking a green tea extract along with a starchy meal (corn flakes) significantly reduced carbohydrate absorption. This may help explain why green tea may help modestly with weight management. Details about this study, including dosage, along with our tests of green tea supplements and teas, are found in the Green Tea Review »
Clinical Update
8/25/2015
Are Green Tea Pills Safe?
Studies have suggested that extracts of green tea can reduce the risk of certain cancers, improve memory, shrink uterine fibroids, and even help with weight reduction. However, liver toxicity has been a concern with green tea extracts. A large, government-funded study recently found that pills of green tea extract can be safe for many people, but some may experience adverse reactions, including elevated liver enzymes. Get the details in the "Concerns and Cautions" section of the Green Tea Review, along with information about what green tea does and our tests of green tea extracts, brewable teas, and beverages >>
CL Answer
With herbal supplements, what is the difference between root powder and root extract? Does it matter?
Learn more about the different forms of herbal supplements, including aloe, boswellia, ginkgo, green tea, and milk thistle.
Clinical Update
4/02/2019
Green Tea and Breast Cancer
Drinking green tea has been associated with a reduced risk of breast cancer recurrence. But what effect does high-dose green tea extract have? See what a major study found in the What It Does section of the Green Tea Review. Also see our Top Picks among green tea products.
Clinical Update
1/18/2014
Green Tea "Blocks" Beta-Blocker
A new study shows that drinking green tea can drastically reduce the amount of a beta-blocker (nadolol, Corgard) absorbed into the bloodstream. Beta-blockers are typically taken to lower blood pressure, raising concern for people using green tea along with this beta-blocker and, possibly, related medications. Get the details, along with our tests of green tea drinks and supplements, in the update to the Green Tea Review >>
If you take a beta-blocker (which includes the popular drugs metoprolol (Lopressor, Toprol) and propranolol (Inderal)), be aware that chromium and CoQ10 may offer benefits.
Clinical Update
1/06/2025
Green Tea Interaction for Men
Green tea may significantly increase blood levels of a common medication for men. Get the details in the Concerns and Cautions section of our Green Tea Review, which includes information about many other green tea interactions, including with statins and blood-thinners.
Also see our Top Picks among green tea beverages and supplements.
Clinical Update
11/21/2023
Green Tea for Blood Cancer?
Does supplementing with green tea improve biomarkers in patients with leukemia, lymphomas or other blood cancers? Find out what research has shown in the Cancer Treatment section of our Green Tea Supplements Review, which includes our Top Picks for brewable tea and green tea supplements.
Clinical Update
7/25/2023
Green Tea for Fibroids?
A preliminary clinical study showed that EGCG from green tea extract may help shrink uterine fibroids. New research suggests why. For details, see the Fibroids section of our Green Tea Supplements Review. Also see our Top Picks among green tea supplements and teas and find out how much EGCG they contain.
Clinical Update
2/14/2023
Green Tea: More EGCG and Less Caffeine?
A CL member asked how steeping green tea in cold water rather than brewing in hot water affects levels of polyphenols and caffeine in the tea. See what a study found in the ConsumerTips section of our Green Tea Review, which includes our Top Picks for green tea.
Clinical Update
6/18/2014
Does Green Tea Really Help With Weight?
A new study found that a popular green tea extract did not cause a reduction in weight compared to placebo, despite the fact that some previous studies have shown a benefit. Get more details, including which green tea extract was used, in the update to the Green Tea Supplements, Drinks and Teas Review >>
Clinical Update
9/28/2013
Green Tea to Treat Fibroids
A new study suggests that green tea extract may help shrink uterine fibroids (common in women of childbearing age) and reduce symptoms. For details, as well as test results and comparisons of green tea extracts and brewable teas and drinks, see the updated Green Tea Review >>
Clinical Update
1/16/2015
Another Use for Green Tea?
A recently published study found that rinsing with green tea reduced plaque-forming bacteria in the mouth just as well as a prescription mouthwash. Get the details, including the form and dose of green tea used, in the Green Tea Supplements, Drinks and Brewable Teas Review >>
Clinical Update
4/22/2015
Green Tea Extract and Prostate Cancer
A new study, funded by the National Institutes of Health, showed that fewer men with pre-cancerous lesions developed prostate cancer if given a daily green tea extract supplement, although the results were not statistically significant due, in part, to the small size of the study. However, a somewhat similar study found a significant benefit. Learn more about these studies, and see our test results for green tea supplements, teas, and drinks, in the Green Tea Review >>
Clinical Update
3/16/2016
Don't Take Green Tea With Iron?
A study shows that iron can bind to a key flavanol (EGCG) in green tea, causing it to lose its anti-oxidant activity. Should you avoid iron-containing foods when using green tea? Find out in the "What to Consider When Using" section of the Green Tea Supplements and Teas Review >>
Clinical Update
1/30/2019
Getting the Most from Green Tea
If you drink green tea to get beneficial compounds like EGCG, learn about a recent study that suggests that the type of water you use to brew tea drastically affects the amount of EGCG you get. Get the details in the How to Take section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
3/30/2019
Green Tea for Lowering Blood Pressure
Drinking green tea can help lower high blood pressure — and even reverse ventricular hypertrophy, according to a new study. For details, including the amount and brand of tea used, see the What It Does section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
8/17/2019
Green Tea and Blood Sugar
Can drinking green tea reduce the rise in blood sugar after a meal, and if so, is the effect greater in the morning or evening? See what a new study found in the What It Does section of the Green Tea Review. Also see our tests and Top Picks among green tea supplements, and brewable and bottled teas.
Clinical Update
6/02/2013
Don't Drink Too Much Tea!
After publication of our Green Tea Review last month, a CL member emailed us about the potential danger of getting too much fluoride from tea. Although this won't happen from just a few cups of tea daily, there have been recent cases where habitually drinking extremely large amounts of tea has caused "fluorosis" causing bone pain and even tooth loss. See the warning (and our tests of green tea products) in the updated Green Tea Review >>
Clinical Update
12/23/2013
Liver Toxicity with Supplements, Including Green Tea
An increase in cases of liver toxicity associated with dietary supplement use has recently been reported. In an article about the findings, The New York Times reports that bodybuilding supplements and products containing green tea extracts are among those associated with such cases. For more information about green tea extracts and toxicity, see the Concerns and Cautions section of the Green Tea Supplements Review >>
Clinical Update
7/06/2016
Lower Risk of Death With Green Tea
A long-term study of men in China found that those who regularly drank green tea — particularly larger amounts — were less likely to die during the study and had lower risks of dying from cardiovascular disease or cancer, compared to non-green tea drinkers. Get the details in the "What It Does" section of the Green Tea Review >>
Clinical Update
7/11/2015
Green Tea for Weight Management?
Green tea has been touted for weight management, but a recent study with a green tea extract casts doubt. More details are found in the "What It Does" section of the Green Tea Review >>
Clinical Update
11/12/2019
Green Tea & Stroke
Studies have found green tea consumption to be associated with a decreased risk of stroke. However, a recent study found this to be the case for only for one gender. For details, see the What It Does section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
3/20/2019
Hot Tea & Cancer
Drinking very hot tea increases the risk of developing esophageal cancer. How long should you wait after brewing to drink tea? Find out what recent studies are showing in the What It Does section of the Green Tea Review. Also see other tips for the best way to prepare brewable green tea, and our Top Picks among green tea products.
Clinical Update
9/24/2019
Does Green Tea Prevent Cognitive Decline?
Does drinking green tea reduce the risk of cognitive decline and, if so, how much do you have to drink? A multi-year study addressed this question. See the results in the Memory and Cognition section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
7/20/2018
Green Tea as a Prebiotic
Green tea may have a prebiotic effect — stimulating the growth of beneficial bacteria in the gut. Find out what clinical trials are showing in the What It Does section of the Green Tea Review. (Also see our Top Picks among green tea products).
Clinical Update
11/14/2017
How Much Tea Is Safe?
Green and black teas contain fluoride, which helps build teeth and bones, but excessive intake of teas can cause teeth and bones to become brittle ("fluorosis"). How many cups of tea are safe? Find out now based on recent tests of fluoride in green, black, and herbal teas in the "Concerns and Cautions" section of the Green Tea Review >>
Clinical Update
6/18/2016
Green Tea & Cognition
Green tea consumption has been associated with a lower prevalence of cognitive impairment in older adults. However, a recent study evaluating the effects of a green tea powder on older adults with Alzheimer's disease and other forms of dementia failed to show a benefit. For more information, see the "What It Does" section of the Green Tea Review >>
Clinical Update
3/07/2018
Statins and Green Tea
If you take a statin drug to lower your cholesterol, be aware that green tea can moderately affect blood levels of certain statins, as noted in a recent report. This effect also depends on the amount of green tea consumed and individual variability. For details see the Concerns and Cautions section of the Green Tea Review.
Clinical Update
9/29/2020
Green Tea for Memory?
Can a green tea extract supplement improve memory or cognitive function in healthy, older men and women? Find out what a recent study showed in the What It Does section of our Green Tea Review. Also see our Top Picks for green tea supplements and drinks.
Clinical Update
9/12/2020
Green Tea and Cholesterol
Can green tea help lower cholesterol levels? Find out what a recent study showed in the What It Does section of Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
2/03/2023
Green Tea & Heart Disease
Find out how many cups of green tea per day is associated with lower risk of heart disease in the What It Does section of our Green Tea Supplements Review, and see our Top Picks for green tea.
Clinical Update
8/22/2023
Green Tea and Blood Sugar
A laboratory study showed how green tea might help control blood sugar. Get the details in the Blood Sugar Control/Diabetes section of our Green Tea Review, which includes our Top Picks for green tea.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
9/01/2023
Green Tea Interactions
Drinking green tea can affect the absorption of many drugs, as was most recently shown with raloxifene (Evista), a medication used to treat osteoporosis and reduce breast cancer risk. Learn about these interactions (including those with certain statins, beta-blockers, and blood thinners) in the Concerns and Cautions section of our Green Tea Review. Also see our Top Picks among green tea products.
Clinical Update
9/12/2023
Green Tea for Cold Sores?
Does green tea help prevent or treat cold sores? Find out what research suggests in our Green Tea Review, which includes our Top Picks for green tea.
Also see: Can any vitamins or minerals help prevent or reduce canker sores or cold sores?
Clinical Update
3/12/2024
Green Tea & Blood Sugar
Does green tea reduce fasting blood sugar levels? See what a recent study showed in the Blood Sugar Control/Diabetes section of our Green Tea Review, which includes our Top Picks for green tea.
Clinical Update
3/22/2024
Lower Cholesterol with Green Tea
Drinking green tea can lower cholesterol or triglyceride levels, as was recently shown. See the details in the What It Does section of our Green Tea Review, which includes our Top Picks for green tea.
Clinical Update
2/07/2017
Colon Polyps and Green Tea
A study of middle-aged men and women found that those given green tea extract after colon polyp removal were much less likely to develop additional polyps than those who did not take the extract. For details about the extract and dose, see the "What It Does" section of the Green Tea Supplements Review (which includes our tests of supplements and teas) >>
Clinical Update
8/04/2023
Chemical Solvents in Tea?
Are chemical solvents used to decaffeinate green tea? Find out in our Green Tea Review, which includes our Top Picks for green tea. Also learn about solvents in coffee.
Clinical Update
2/21/2024
Forever Chemicals in Tea?
A recently published study found an association between intake of "unsweetened" tea and higher blood levels of PFAS ("forever chemicals"). Should you be concerned? See the details in the Concerns and Cautions section of our Green Tea Review, which includes our recent Top Picks among products.
Also find out if "plant-based" plastic tea bags may be less likely to release microplastics than regular plastic or nylon tea bags.
A new video explaining how to choose the best green tea is also now available.
Clinical Update
2/24/2025
Plastic in Bigelow Tea Bags?
Due to concern over microplastics, we asked Bigelow if their tea bags contain plastic. It turns out that some do and some don’t, as we explain in the Concerns with Plastic Tea Bags section of our Green Tea Review, which includes similar information for other tea brands. The Review also includes our Top Picks among green teas and supplements.
Clinical Update
3/10/2025
Plastic in Twinings or Tazo Tea Bags?
We asked Twinings and Tazo if their tea bags contain plastic. See their responses in the Concerns with Plastic Tea Bags section of our Green Tea Review, which includes similar information for other tea brands. The Review also includes our Top Picks among green teas and supplements.
Clinical Update
3/17/2025
Plastic in Numi Tea Bags?
We asked Numi if their tea bags contain plastic. See their response in the Concerns with Plastic Tea Bags section of our Green Tea Review, which includes similar information for other tea brands. The Review also includes our Top Picks among green teas and supplements.
Clinical Update
2/27/2025
Lead & Tea
Although some teas are contaminated with lead (as we have shown), a recent study demonstrated that tea can also remove lead from the water in which is it brewed or steeped. Get the details in the Brewing section of our Green Tea Review, which includes our Top Picks among green teas.
Clinical Update
1/13/2025
Plastic from Tea Bags
A small study suggested that some plastic tea bags release much larger amounts of nanoplastics than others. For details, and how to avoid nanoplastics from tea bags, see our Green Tea Review, which includes our Top Picks for green tea.
Clinical Update
1/27/2024
Green Tea Interaction With Medicine
Drinking green tea may significantly affect blood levels of a common beta-blocker, as well as statins, blood-thinners, and thyroid medication. Be aware of these interactions. See the Concerns and Cautions section of our Green Tea Review.
Clinical Update
9/25/2011
Green Tea Extract and Weight Loss
A new study using a high dose of green tea extract showed it to help sedentary overweight men lose weight, while those taking placebo gained weight. This is not the first study to show this potential benefit of green tea extract. The effect may be due to the compound EGCG. Get the facts, including how much EGCG was in the product, in the Weight Loss Supplements Review. More >>
Clinical Update
Clinical Update
1/14/2020
Tea & Heart Disease
Another study shows that people who drink tea are less likely to suffer from cardiovascular disease. Find out how much and what type of tea was associated with benefits in the Cardiovascular disease section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
4/24/2018
Avoiding Green Tea Toxicity
The European counterpart to the FDA recently issued its conclusions regarding toxicity from green tea catechins. Get the details in the Concerns and Cautions section of the Green Tea Review.
Clinical Update
10/06/2017
Green Tea & Prostate Cancer
A recent study investigated the effects of green tea extract in men with pre-cancerous changes of the prostate. Find out if it helped in the "What It Does" section of the Green Tea Supplements and Drinks Review >>
Clinical Update
4/27/2019
No Ginseng In Ginseng Tea?
A popular maker of bottled teas is being sued for allegedly not putting ginseng in its ginseng green tea. For details, see the Quality Concerns section of the Ginseng Supplements Review. Also see which ginseng products were Approved by ConsumerLab (and see our tests of various Green Teas).
Clinical Update
3/14/2023
Are Plastic Tea Bags Okay?
Are plastic (nylon) tea bags okay to use? There are two potential problems, as we explain in the ConsumerTips section of our Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
9/02/2022
Black Tea for Heart Health?
Drinking green tea has been linked with reduced risk of death due to heart disease, but how about black tea? See what a recent study found in the Cardiovascular Disease section of our Green Tea Supplements and Drinks Review.
CL Answer
Can any vitamins or minerals help prevent or reduce canker sores or cold sores?
Find out which, if any, supplements, including iron, L-lysine, monolaurin, olive leaf extract, vitamin B-12, and zinc, may be beneficial for canker sores or cold sores.
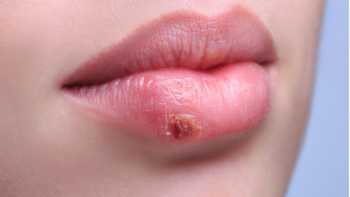
CL Answer
I take warfarin (Coumadin), an anticoagulant drug. Are there supplements I should avoid, or be taking, due to this drug?
Find out which supplements should be avoided when taking blood thinners (anticoagulants) like warfarin (Coumadin), including vitamin K, St. John's wort, and curcumin.

CL Answer
Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Find out which supplements help improve memory, brain function and cognition, including fish oil, some B vitamins, cocoa, and curcumin. ConsumerLab's answer explains the evidence for supplements promoted to help with brain function and cognition.
CL Answer
What is Protandim and does it really work?
What is Protandim and does it really work? Can Protandim reduce oxidative stress? ConsumerLab's answers discusses Protandim's effectiveness, reviews and studies.

Clinical Update
2/10/2025
Avoiding Microplastics from Tea Bags
Recent studies have raised concerns about microplastics released from plastic tea bags. To avoid plastic tea bags, see the Results table in our Green Tea Review, which lists the type of tea bag (paper, plastic, or mixed) for each of the bagged teas we’ve tested. Also see our Top Picks among brewable teas and a list of tea companies that state they do not use plastic in their tea bags.
Clinical Update
4/21/2025
Are Plastics in Tea Bags Safe?
A plant-based plastic known as PLA is used in some tea bags, but a new study in animals suggests ingesting PLA might have adverse effects. Get the details in the Concerns With Plastic Tea Bags section of our Green Tea Review, which identifies which tea bags include plastic, and which tea companies state they do not use plastic, and see our Top Picks among brewable teas.
CL Answer
Can fluoride in toothpaste, mouth rinses or drinking water cause attention-deficit/hyperactivity disorder (ADHD) or other neurodevelopmental disorders in children?
Find out if community water fluoridation increases the risk of attention-deficit/hyperactivity disorder (ADHD) or causes lower IQ in children, and learn what the American Dental Association recommends when using fluoride-containing toothpaste and mouth rinses in children.

CL Answer
Is it safe to drink coffee regularly? Does drinking coffee increase or decrease the risk of cancer?
Learn more about coffee, its safety, if it's good for you, and if it impacts your risk of cancer.

CL Answer
I read that sterol supplements to lower cholesterol like CholestOff can block the absorption of vitamins. Is that true?
Find out if CholestOff and other sterol supplements can block vitamin absorption in the body and other safety concerns.

Clinical Update
10/14/2024
Rooibos Tea?
What are the potential health benefits of rooibos tea, how does it compare to black and green tea, and is it safe? Find out in our article about rooibos tea.
Clinical Update
10/21/2024
Benefits of Mullein Tea?
What is mullein tea and does it really reduce coughing, or help ward off the flu or other viruses? Find out in our new article about the potential health benefits and safety of mullein tea.
Also see our Green Tea Review, including our Top Picks.
Clinical Update
3/18/2017
Tea Drinking Associated with Less Cognitive Decline
A new study found tea drinkers to have significantly less cognitive decline than others. Read the details in the "What It Does" section of the Green Tea Supplements Review (which includes our tests of teas) >>
Clinical Update
3/27/2025
Plastic in Trader Joe's Tea Bags?
We asked Trader Joe's if there is plastic in its green tea bags. See Trader Joe's response as well as those of Bigelow, Numi, Republic of Tea, Tazo, and Twinings.
Recalls & Warnings
October 09, 2025
Lipton Green Tea Citrus Recalled
On September 17, 2025, PepsiCo, Inc.
Clinical Update
8/25/2023
Supplements for Memory
Find out if ingredients such as apoaequorin (found in Prevagen), ashwagandha, coffee extract, ginger, green tea, phosphatidylserine, or vitamin E, help improve memory and cognition in our article about supplements and foods for memory.
Clinical Update
10/05/2019
L-Theanine for Sleep and Cognition?
Several studies have evaluated whether a single dose of L-theanine (found in green tea) can reduce stress and anxiety. A new study has evaluated the effect of L-theanine when taken daily for several weeks. See the results in the What It Does section of the L-Theanine Supplements Review. Also see our top choices for L-Theanine.
Recalls & Warnings
November 28, 2022
Seller of Elderberry, Tea Warned for Claims of Treating Cold, Flu, Cancer
On October 18, 2022, the FDA issued a warning letter to Rosebud’s Ranch and Garden, LLC after inspection of the company’s website and social media found statements about the company’s Domestic Divas – Colds and Flu (Tea), Domestic Divas - No Pain No Gain Tea, Green ...
Recalls & Warnings
October 09, 2023
Organifi Settles Charges of Unsupported Claims
Organifi, LLC has agreed to pay $150,000 in civil penalties and $50,000 in investigative costs as restitution to settle charges that the company made unsupported claims that its Gold, Pure, Green Juice, and Red Juice products could balance hormone and cortisol levels, regulate the ...
Recalls & Warnings
July 18, 2017
Oat Cereal Recalled Due to Listeria Risk
On July 18, 2017, Garden of Light, Inc., recalled Woodstock Organic Matcha Vanilla Oats because of potential contamination with Listeria monocytogenes.
News Release
February 24, 2018
Magnesium, Curcumin, and Apple Cider Vinegar Rise in Popularity, While Probiotics Dip and Calcium Continues to Decline in Latest ConsumerLab.com Survey of Supplement Users
White Plains, New York, February 24, 2018 — A recent survey of 11,446 people who use dietary supplements shows that magnesium and curcumin and turmeric have leapt into the top 5 most popular dietary supplements, just behind vitamin D, fish oil and CoQ10.
News Release
October 14, 2015
Is Matcha a Better Form of Green Tea? ConsumerLab.com Answers the Question
White Plains, New York, October 14, 2015 — For the past three years, ConsumerLab.com has been testing green tea products, including tea bags, bottled drinks, dietary supplements, and even a K-Cup®.
News Release
July 07, 2014
Which Weight Loss Supplements Are Best? ConsumerLab.com Reviews the Evidence and Tests the Quality of Popular Products
White Plains, New York, July 7, 2014 — Dozens of supplement ingredients have been touted for weight loss, but which have the strongest evidence showing they work and, among those, which products are highest in quality? To answer these questions, ConsumerLab.
News Release
May 21, 2013
Green Teas Vary in Strength and Amount of Lead Contamination, According to ConsumerLab.com
White Plains, New York, May 21, 2013 — If you drink green tea for your health, be aware that the catechin and caffeine levels can vary by more than 240% across products. Some also contain significant amounts of lead in their tea leaves. This is according to recent tests by ConsumerLab.
News Release
December 21, 2012
ConsumerLab.com Tests Reveal What's Really In Green Tea Supplements and Bottled Drinks
White Plains, New York — December 21, 2012 — How do levels of EGCG and caffeine in green tea supplements and bottled teas compare with levels provided in a typical brewed green tea? It very much depends on the brand, according to new tests by ConsumerLab.com.
News Release
February 05, 2012
Fish oil and multivitamins most popular supplements in ConsumerLab.com survey -- Internet most popular place to shop
WHITE PLAINS, NEW YORK — FEBRUARY 5, 2012 — A survey of over 10,000 savvy consumers of supplements shows the most popular supplements to be fish oil, multivitamins, vitamin D, calcium and CoQ10, in that order.
Recalls & Warnings
August 07, 2025
Herbalife “Relaxation Tea” Recalled
On July 21, 2025, Herbalife International of America recalled 5,888 units of its Relaxation Tea because an incorrect ingredient was received from the supplier and used in the manufacturing ...
Recalls & Warnings
April 25, 2024
Yogi Tea Recalled Due to Pesticides
On April 19, 2024, Yogi Tea issued a recall of over 54,800 packs of Yogi Echinacea Immune Support tea after a sample of echinacea tested above acceptable limits for multiple pesticides. No illnesses have been reported to date.
Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
March 01, 2023
Seller of Dr. Miller’s Herbal “Detox” Teas Warned by FDA for Drug Claims
On February 3, 2023, the FDA issued a warning letter to Jackson Health & Wellness Clinic after inspection of the company’s website found statements about the company’s Dr. Miller’s Holy Tea, Dr. Miller’s Super Holy Tea, Dr. Miller’s Ultimate Tea, Dr.
Recalls & Warnings
March 02, 2022
Seller of Holistic Tea Warned by FDA
On February 17, 2022, the FDA issued a warning letter to Vital Health & Wellness following an inspection of the company’s website, which found statements made about the company’s product, Dr. Miller’s Holistic Premium Holy Tea, to be drug claims.
Recalls & Warnings
September 25, 2023
FDA Warns Consumers Not to Use Tapee Tea
On August 31, 2023, the FDA warned consumers not to purchase or use Tapee Tea products after FDA laboratory analysis found them to contain dexamethasone and piroxicam, drugs that are not listed on the product's label.
News Release
March 02, 2010
ConsumerLab.com finds carcinogenic form of chromium in supplements, including those for weight loss -- Reviews published of supplements containing chromium, green tea, 7-keto DHEA and stimulant formulas
White Plains, New York — March 2, 2010 — A carcinogenic form of chromium, hexavalent chromium, is present is some dietary supplements, according to new tests by ConsumerLab.com.
News Release
April 28, 2009
40% of Green Tea and Selenium Products Fail ConsumerLab.com Review of Cancer-Prevention Supplements; Lycopene Supplements Pass
WHITE PLAINS, NEW YORK — APRIL 28, 2009 — ConsumerLab.com announced results today of its Product Review of Supplements for Cancer Prevention. Among the products that ConsumerLab.com selected for testing, quality problems were found with two out of five green tea supplements.
Recalls & Warnings
July 12, 2023
FDA Warns Company for Promoting Kratom Products for Pain, Mood, and More
On July 3, 2023, the FDA issued a warning letter to Sunshine Trading Company, Inc.
News Release
April 26, 2006
ConsumerLab.com tests cancer-prevention supplements; several contaminated with lead or low in ingredient — Report available for green tea, selenium, and lycopene supplements
WESTCHESTER COUNTY, NEW YORK — APRIL 26, 2006 ConsumerLab.com announced results today from its new Product Review of Supplements for Cancer Prevention covering 22 products. Cancer is the second leading cause of death in the U.S.
News Release
November 15, 2005
Testing by ConsumerLab.com identifies many problems with popular supplements for weight loss, slimming, and blood sugar control
Westchester, NY — November 15, 2005 — In a series of reports released today, ConsumerLab.com revealed test results for supplements used for weight loss, slimming, and blood sugar control. Among the twenty-three products that ConsumerLab.
Recalls & Warnings
July 16, 2024
Infla-650 Herbal Dietary Supplement Recalled
On July 16, 2024, Guru Inc. issued a recall of one lot of Infla-650 Herbal Dietary Capsules because they were found to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
November 04, 2015
Green Tea from Tea Bags Linked to Acute Hepatitis
According to a case report published on September 23, 2015 (Lugg, BMJ Case Rep 2015), a 16-year old girl in England developed acute hepatitis in connection with the consumption of green tea.
Recalls & Warnings
January 18, 2019
Another Green Tea Study Retracted
A study suggesting that green tea catechins reduce the invasive potential of human melanoma cells was recently retracted by the journal PLos One on December 31, 2018 due to concerns over the accuracy of the data.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
December 20, 2021
Pantene, Herbal Essences, & Other Brands of Dry Shampoo and Conditioner Recalled Due to Benzene
On December 17, 2021, Procter & Gamble issued a recall of 26 aerosol spray dry shampoos and conditioners from six brands, including Aussie, Herbal Essences, Hair Food, Old Spice, Pantene, and Waterless Hair Care, because some of the products were found to contain benzene.
Recalls & Warnings
August 14, 2020
The Green Herb and New Genesis Health Warned for Manufacturing Violations
On July 31, 2019, the FDA issued a warning letter to Davis Ventures, Inc.
Recalls & Warnings
April 14, 2020
Teami Tea Settles Charges of Unproven Claims and Deceptive Celebrity Endorsements
On March 6, 2020, the FTC announced that Teami, LLC, a marketer of tea and skin care products, agreed to settle charges that it was selling products with unproven claims and misleading celebrity endorsements.
Recalls & Warnings
July 15, 2019
Mislabeled Green Tea Supplement Recalled
On July 11, 2019, DaVinci Laboratories of Vermont issued a recall of 256 bottles of the weight management supplement Dim Plex because they may undeclared fish allergens.
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
November 21, 2017
Health Canada Calls for Stronger Warnings of Liver Risk on Green Tea Extract Products
On November 15, 2017, Health Canada (the Canadian equivalent of the FDA) recommended stronger warning statements about the risk of liver toxicity on labels of supplements containing green tea extract sold in Canada.
Recalls & Warnings
February 07, 2011
Maker of Hoodia and Green Tea Products to Pay $2.65 Million Settlement for Unfair Business Practices
On February 1, 2011, supplement maker Irwin Naturals agreed to pay civil penalties, consumer restitution, and and investigative costs to settle a suit in California alleging that Irwin Naturals marketed Hoodia products that did not contain the hoodia gordonii herb despite the product labeling.
Recalls & Warnings
June 27, 2007
Warning Labels to be Required for Green Tea and Black Cohosh Products
On June 26, 2007, the U.S. Pharmacopeia (USP) Dietary Supplements Information Expert Committee (DSI-EC) voted to require cautionary statements on the labels for green tea extracts and black cohosh dietary supplements.
Recalls & Warnings
May 02, 2016
The Republic of Tea Recalls Turmeric/Ginger Teas Due to Salmonella Risk
On April 29, 2016 The Republic of Tea issued a recall of several Organic Turmeric Ginger teas because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
January 24, 2020
Fat-Burning, Energy Supplement Linked to Heart Trouble
A 33-year-old woman in Australia developed cardiac ischemia (a lack of blood flow to the heart) after taking the "fat burning" supplement Alpha Lean-7, according to a recent report in the Journal of Sports Sciences.
Recalls & Warnings
November 04, 2016
Liver Injuries Linked With Dietary Supplement Use on the Rise
The number of liver injuries associated with dietary supplement use has increased substantially in recent years, according to a recently published study in the journal Hepatology.
Recalls & Warnings
March 15, 2016
Tea Recalled Due to Salmonella Risk
On March 11, 2016 Awareness Corp. issued a recall of its 7.4 ounce container of Boost Tea because it may be contaminated with Salmonella.
Recalls & Warnings
December 19, 2013
Seller of Sexual Enhancement Supplement and Tea Drink Warned for Drug Ingredients and Drug Claims
On December 5, 2013, the FDA issued a warning letter to Green Planet, Inc. following a facility inspection which found the company's sexual enhancement supplement Night Bullet to contain the drugs sulfohydroxyhomosildenafil, thioaildenafil and aminotadalafil.
Recalls & Warnings
January 16, 2014
Seller of Tea Supplements and Drinks Warned For Manufacturing Violations and Drug Claims
On December 31, 2013, the FDA issued a warning to Prestige Chinese Teas Company, Inc.
Recalls & Warnings
May 06, 2017
Herbal Teas Recalled Due To Botulism Risk
On May 1, 2017, U.S. Deer Antler Ex. & Imp. of Los Angeles, California, issued a recall of various herbal teas because they have the potential to be contaminated with Clostridium botulinum. This bacterium can cause botulism, a potentially fatal form of food poisoning.
Recalls & Warnings
January 11, 2013
Consumers May Be Eligible For Cold, Allergy and Weight Loss Supplement Refunds
On January 7, 2013, the Federal Trade Commission (FTC) announced that consumers who purchased the Iovate Health dietary supplements Accelis, nanoSLIM, Cold MD, Germ MD, or Allergy MD between January 2006 and July 2010 may qualify for a refund.
Recalls & Warnings
January 10, 2013
FDA Warns Aloe and Herbal Supplement Companies For Manufacturing Violations
AloeScience Labs, Inc. -- On November 14, 2012, the FDA issued a warning letter to AloeScience Labs, Inc.
Recalls & Warnings
December 07, 2012
Antioxidant Protandim Supplement Recalled Due To Risk of Metal Fragments
On December 5, 2012, LifeVantage Corporation issued a voluntary recall of dietary supplement Protandim, the Nrf2 Synergizer, due to the potential for contamination with small metal fragments. The recall includes Protandim products sold in the U.S. and Japan between July and November 2012.
Recalls & Warnings
February 15, 2013
Energy Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of the energy supplement MET-Rx Extreme Thermo Rage Watermelon 8 fl. oz. (Manufactured by MET-Rx Nutrition, Inc.) due to bacterial contamination.
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
September 16, 2015
Herbal Extracts Recalled
On September 15, 2015, Iowa Select Herbs, LLC issued a recall of numerous herbal exacts following a permanent injunction which required the company to stop selling supplements.
Recalls & Warnings
August 28, 2013
Tea and Papaya Supplement Company Warned For Drug Claims
On August 12, 2013, the FDA issued a warning letter to Herbal Papaya, LLC, following a review of the company's website and Facebook page which found statements made about Papaya Seed Extract Capsules, 100% Papaya Leaf (Paw Paw Twig) and Papaya Leaf with Rooibos Tea to be drug claims.
Recalls & Warnings
September 13, 2014
Marketer Banned From Selling Weight Loss Products
In order to settle FTC charges of deceptive weight loss claims, John Matthew Dwyer III, the co-founder of HealthyLife Sciences, LLC, has agreed to no longer manufacture or market weight loss supplements.
Recalls & Warnings
June 12, 2014
Marketers of Memory Supplement Settle FTC Charges of Deceptive Claims
Martek Biosciences Corporation and i-Health Inc., marketers of BrainStrong Adult, have agreed to settle Federal Trade Commission (FTC) charges that claims the supplement could improve adult memory and prevent cognitive decline were deceptive, and not supported by sufficient clinical evidence.
Recalls & Warnings
May 24, 2013
Brewable Tea and Tea Supplement Company Warned For Manufacturing Violations and Drug Claims
On April 2, 2013, the FDA issued a warning letter to Natures Health Options, LLC, following an investigation which found the company's Charantea Bitter Melon Ampalaya, which is distributed as a dietary supplement in capsule form, and as a tea, to be adulterated because it was prepared, packed, or ...
Recalls & Warnings
February 28, 2017
Seller of Tea Supplements Warned for Manufacturing Violations
On February 3, 2017, the FDA issued a warning letter to Kumato Labs, following a facility inspection which found the company's products, including CHO WA Tea, COMOXIN IBS Formula and NEMURI Sleep Formula to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
January 21, 2014
"Stem Cell" Supplement Recalled Due to Allergen Risk
On January 19, 2014, Stemvida recalled StemAlive 90 Capsules because they were found to contain undeclared milk (labeled as bovine colostrum).
Recalls & Warnings
January 24, 2013
Food for Health Warned for Manufacturing Violations and Drug Claims on Supplements
On October 5, 2012, the FDA issued a warning letter to Food for Health International, LLC following a facility inspection which found the company's products, including Activz brand Vitamin D, Potassium Iodine, Organic Vitamin C, Whole 9 (a fruit and vegetable meal replacement shake) Control and VMA ...
Recalls & Warnings
March 06, 2015
Seller of Tea Drinks Warned for Drug Claims
On February 26, 2015, the FDA issued a warning letter to the seller of Anamu CancerHerb Tea Box, Anamu CancerHerb Tea Box with Honey and Organic Bottled Tea Drinks because statements made about these products on the websites, www.cancerherbtea.com and www.anamucancerherbtea.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
April 26, 2013
Seller of Immune, Cholesterol, Liver and Blood Sugar Products Warned For Drug Claims
On March 20, 2013, the FDA issued a warning letter to Birkdale Medicinals LLC, following a review of the company's website which found statements made about Birkdale products, including Immune Response 247, Cholestat, Silymarin 81 and LevelStat, to be drug claims.
Recalls & Warnings
April 26, 2018
Wegmans Recalls Traditional Medicinals Teas Due to Salmonella Risk
On April 17, 2018, Wegmans Food Markets issued a recall of one lot each of Traditional Medicinals Throat Coat Lemon Echinacea herbal tea and Traditional Medicinals EveryDay Detox Lemon tea because they have the potential to contaminated with Salmonella.
Recalls & Warnings
October 24, 2012
Manufacturer of Green Tea, Vitamin E, Omega-3 and Cranberry Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On October 16, 2012, the FDA issued a warning letter to dietary supplement manufacturer Advanced Nutritional Technology Inc.
Recalls & Warnings
April 24, 2018
Traditional Medicinals "Throat Coat" Lemon Echinacea Herbal Tea Recalled in Canada
On April 24, 2018, Health Canada (the Canadian equivalent of the FDA) advised consumers that one lot of Traditional Medicinals Throat Coat Lemon Echinacea herbal tea is being recalled in Canada because it has the potential to contaminated by Salmonella.
Recalls & Warnings
June 23, 2018
Gaia Kratom Recalled Due to Salmonella Risk
On June 21, 2018, Gaia Ethnobotanical, LLC issued a recall of 27 kratom products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
June 19, 2018
FDA Warns Seller of Weight Control Patches
On June 6, 2018, the FDA issued a warning letter to Health Management Group, Inc.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
November 30, 2013
"Diuretic" Weight Loss Supplement Recalled
On November 29, 2013, IQ Formulations issued a recall of all lots of diuretic weight loss supplement HYDRAVAX because one lot of the supplement was found to contain an undeclared prescription diuretic drug.
Recalls & Warnings
February 13, 2019
Supplements Promoted for Alzheimer's Disease and Dementia Sell "False Hope," Warns FDA
On February 11, 2019, the FDA warned consumers to beware supplements promoted to prevent or treat Alzheimer's disease or dementia.
Recalls & Warnings
January 02, 2012
FDA Warns XYMOGEN of Manufacturing and Labeling Violations Regarding Multiple Products
On December 13, 2011, the U.S. FDA sent a Warning Letter to Atlantic Pro Nutrients, Inc. (dba XYMOGEN) regarding labeling and/or manufacturing violations relating to its products, which include Borage CP-240, CoQmax CF, Immune Rx, Iron Glycinate, and Green Tea 600.
Recalls & Warnings
May 08, 2018
Badger Botanicals Kratom Recalled
On May 4, 2018, 2018 Badger Botanicals issued a recall of its Green Suma, Red Suma, Green Hulu 2, and Red Hulu 2 kratom dietary supplements because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
August 21, 2013
Seller of Vitamin, Tea and MSM Supplements Warned For Drug Claims
August 7, 2013, the FDA issued a warning letter to Vibrant Life Vitamins, following a review of the company's websites which found statements made about Taheebo Life Tea, Life Glow Plus, Super Life Glow, Life Glow Basic, Vibrant Life MSM, Herbal MSM, and Organic Germanium to be drug claims.
Recalls & Warnings
August 20, 2013
Weight Loss Supplement Formulas For Men and Women Recalled Due To Undeclared Drugs
On August 16, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss dietary supplements Esbelder man, Esbelder fem and Esbelder siloutte because they were found to contain the undeclared drugs sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
August 01, 2013
Fat Loss and Muscle Enhancement Supplements Found To Contain DMAA
On June 17, 2013, the FDA issued a warning letter to Formulife, Inc., dba Purus Labs, Inc., following a facility inspection which found the dietary supplements Fat Smack XR Thermolipolytic, Muscle Marinade Fresh Fruit and Muscle Marinade Cherry Limeade to contain DMAA.
Recalls & Warnings
December 06, 2013
Weight Loss Supplement Claims Challenged
On November 27, 2013, the National Advertising Division (NAD) recommended HealthyLife Sciences, LLC, modify or discontinue the use of certain claims made about the company's weight loss supplement Healthe Trim.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
February 21, 2013
FDA and FTC Warn: Supplements Cannot Prevent, Treat Or Cure Cold And Flu
On February 11, 2013, the FDA and the Federal Trade Commission (FTC) jointly issued warning letters to three dietary supplement companies regarding their potential illegal marketing of products to prevent, treat or cure flu virus.
Recalls & Warnings
September 23, 2014
Maker of Joint and Weight Products Warned for Hidden Drugs, Manufacturing Violations
On September 15, 2014, the FDA issued a warning letter to West Coast Laboratories, Inc., because the company's joint health supplements, Super ArthGold and Pro ArthMax, were found to contain hidden drugs.
Recalls & Warnings
August 16, 2018
Kratom Recalled Due to Salmonella Risk
On August 14, 2018, Zakah Life, LLC issued a recall of Super Green Maeng Da Premium Kratom powder, Powerful Red Vein Bali Premium Kratom powder, Super Green Maeng Da Premium Kratom capsules, and Powerful Red Vein Bali Premium Kratom capsules because laboratory testing revealed the presence of ...
Recalls & Warnings
February 28, 2013
Maker of Liquid Omega-3 Supplements Warned For Manufacturing Violations
On February 20, 2013, the FDA issued a warning letter to Capco Custom Packaging Inc.
Recalls & Warnings
April 04, 2013
FDA Warns Consumers Of Weight Loss Supplement Containing Undeclared Drug
On April 4, 2013, the FDA warned consumers not to purchase or use the dietary supplement MAXILOSS Weight Advanced Blue because it was found to contain sibutramine.
Recalls & Warnings
March 21, 2013
Probiotic Recalled Due To Undeclared Soy
On March 20, 2013, New Chapter, Inc. issued a voluntary recall of one lot of its Probiotic Elderberry dietary supplement because it may contain undeclared soy.
Recalls & Warnings
September 12, 2018
FDA Urges Consumers to Avoid Kratom
On September 11, 2018, FDA Commissioner Scott Gottlieb, M.D., urged consumers not to use products containing the kratom, an herb that is often promoted for pain relief and for relieving symptoms of opioid withdrawal.
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.
Recalls & Warnings
October 24, 2012
FDA Seizes Many Supplements from New York Company Due To Drug Claims
On October 23, 2012, U.S. Marshalls, acting on behalf of the FDA, seized dietary supplements and unapproved drugs from Confidence, Inc., a supplement manufacturer in Port Washington, N.Y. The products included dietary supplements Dr.
Recalls & Warnings
April 03, 2018
FDA Issues Mandatory Recall of Salmonella-Contaminated Kratom Products
On April 2, 2018, the FDA issued a mandatory recall of all food products containing powdered kratom manufactured, processed, packed, or held by Triangle Pharmanaturals LLC because several of company's products were found to be contaminated with Salmonella.
Recalls & Warnings
April 02, 2018
NutriZone Kratom Supplements Recalled Due to Salmonella Risk
On March 10, 2018, NutriZone, LLC issued a recall of certain kratom-containing powder products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
January 04, 2007
Sellers of Popular Weight Loss Supplements Pay $25 Million Over FTC Allegations of Deceptive Advertising
On January 4, 2007, the Federal Trade Commission (FTC) announced that it had filed complaints in four separate cases alleging that weight-loss and weight-control claims were not supported by competent and reliable scientific evidence.
Recalls & Warnings
January 10, 2018
Lithium Supplement Contaminated With E. Coli, Health Canada Warns
On December 1, 2018, Health Canada (the Canadian equivalent of the FDA) warned consumers that it found Smart Brain Formulations Serotonin Support, a lithium ororate supplement sold on the website Robert Lamberton Consulting, to be contaminated with E. choli (Escherichia coli).
Recalls & Warnings
November 12, 2017
Weight Loss and Cleanse Supplements Contain Adulterated and Improperly Labeled Ingredients, FDA Warns
On November 2, 2017, the FDA issued a warning letter to Create-A-Pack Foods, Inc.
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
August 06, 2016
Some Supplements May Cause or Exacerbate Heart Failure (Includes vitamin E and many herbs)
Taking certain supplements may be dangerous for people with heart failure, according to a statement from the American Heart Association (AHA) which was published in the journal Circulation (August 2, 2016).
Recalls & Warnings
September 27, 2013
Company Warned For Distributing Weight Loss Supplement Containing DMAA, Drug Claims
On September 6, 2013, the FDA issued a warning letter to Pure Energy Products, Inc., following a facility inspection which found that the company distributes a weight loss supplement, called obestrim, which contains dimethylamylamine, or DMAA.
Recalls & Warnings
March 03, 2012
Seller of Cancer Cures Warned by FDA
On February 9, 2012, the U.S. FDA sent a Warning Letter to BioAnue Laboratories, Inc. informing it that its websites at www.tumorx.com, www.cancerx.org, www.hopewelltechnologieslimited.com, and www.vmhe.
Recalls & Warnings
January 31, 2002
Recall Update for Certain Metabolife Bars - Excessive Vitamin A
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 30, 2002 Enforcement Report.
Recalls & Warnings
September 10, 2012
Maker of Omega-3, Bone and Brain and Supplements Warned For Drug Claims
On August 27, 2012, the FDA issued a warning letter to PruTect Rx indicating that statements on the company's websites about Omega3PruTect, NeuroPruTect, VitaminD3PruTect and OsteoPruTect constitute drug claims, although the products are not approved drugs.
Recalls & Warnings
February 25, 2013
Weight Loss Supplement Recalled Due To Undeclared Drug
On February 21, 2013, Olaax Corp. issued a voluntary, nationwide recall of the company's weight loss supplement MAXILOSS Weight Advanced softgels because they were found to contain undeclared Sibutramine.
Recalls & Warnings
February 14, 2013
Maker of Antioxidant and Anti-Aging Supplements Warned For Manufacturing Violations
On January 10, 2013, the FDA issued a warning letter to dietary supplement manufacturer Consolidated Marketing Unlimited, Inc.
Recalls & Warnings
February 13, 2013
FDA Warns Maker of Weight Loss and Whey Protein Supplements For Manufacturing Violations and Misbranding
January 25, 2013, the FDA issued a warning letter to dietary supplement manufacturer SciLabs Nutraceuticals, following a facility inspection which found the company's dietary supplements and supplement ingredients, including N-Large 2, Flo-Gard AB, Nitrogen Glutamine capsules, Nutritech nutritional ...






