Product Reviews and Information for High Blood Pressure (hypertension)
Search term may appear only in full report available to members. Join now for full access.
Product Review
Potassium Supplements Review
Be Careful with Potassium Supplements! Problems Found. Tests and Reviews of Potassium Supplements & CL's Top Picks.
Product Review
Taurine Supplements Review for People, Dogs, and Cats
Find the Best Taurine Supplements. Learn When to Use Taurine and Which Supplement is Best.
Product Review
GABA Supplements Review
Does GABA Work As a Supplement?

Product Review
Toprol XL vs. Generic Metoprolol Succinate Extended-release (ER) Tablets Review Article
Choose the Best Blood Pressure Medication. Find Out Why Some Generic Blood Pressure Medications Are Not the Same as the Original & May Increase Blood Pressure or Have Other Disturbing Side Effects.
CL Answer
I have low blood pressure. Are there any supplements I should avoid?
Find out which supplements can cause decreases in blood pressure, including melatonin, arginine, magnesium, and calcium.

Product Review
Acetyl-L-Carnitine Supplements Review
Choose the Best Acetyl-L-Carnitine Supplement. Don't Overpay! CL Identifies Quality Acetyl-L-Carnitine Supplements at the Best Value.
.jpg?size=small)
Product Review
Nattokinase Supplements Review
Choose the Best Nattokinase Supplement. CL Tests Reveal Which Nattokinase Suppplements Provide the Best Quality & Value.
Product Review
Pomegranate Juice and Supplements Review Article
What Are the Benefits of Pomegranate Juice and Supplements? Find Out What Pomegranate Can and Cannot Do For Your Health.

CL Answer
Which supplements can help to lower blood pressure?
Supplements to lower blood pressure, including CoQ10, vitamin C, fish oil, and olive oil, are all explored to find out what works.

Product Review
Garlic Supplements Review
Find the Best Garlic Supplements. CL Tests Reveal Big Differences in Garlic Strength -- Some Have Little to No Garlic!.

Product Review
Magnesium Supplements Review (Including Calcium, Vitamins D & K, and Boron)
Find Out What Magnesium Does, Who Needs It, and Our Top Picks Among Supplements
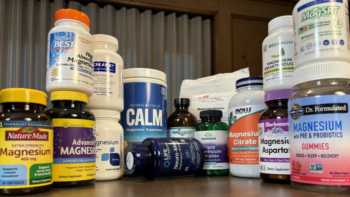
CL Answer
I take lisinopril (Zestril), an ACE inhibitor drug to lower blood pressure. Are there supplements I should avoid, or be taking, due to this drug?
When taking ACE inhibitors, find out which supplements should be avoided. Interaction info on Lisinopril or another ACE inhibitors.
Product Review
Weight Loss Supplements Review (7-Keto DHEA, Forskolin and Stimulant Blend Supplements)
Choose the Best Weight Loss Supplement. Be Careful With Weight Loss Supplements — Few Pass Quality Testing and Safety Review.

CL Answer
Can beetroot juice or supplements lower blood pressure or improve exercise performance, and are they safe?
Learn more about beetroot juice and beetroot supplements and if beetroot can really help lower blood pressure.

Product Review
Black Currant, Borage, Evening Primrose, Flax and Hemp Oil Review
Choose the Best Flaxseed and Other Oils Based on Our Tests.

Product Review
Vitamin C Supplements Review
Find the Best Vitamin C Supplements

Product Review
Vitamin D Supplements Review (Including Calcium, Magnesium, Vitamin K, and Boron)
Find the Best Vitamin D Supplement and Avoid Problems

Product Review
Hoodia (Hoodia gordonii) Supplements Review Article
Hoodia Supplements for Weight Loss. Will Hoodia help you lose weight? Make sure you know the facts.
Product Review
L-Citrulline Supplements Review
Learn How Citrulline Products Differ and Our Top Picks

CL Answer
A tea called Throat Coat made me lethargic and I was found to have low potassium. Is this a known problem? I was drinking 5 cups a day.
Throat Coat tea information, including licorice root content, potassium loss, and symptoms such as lethargy, increased blood pressure and fluid retention. ConsumerLab.com's answer explains.

Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

Product Review
Lycopene Supplements Review
Find the Best Lycopene Supplements. CL Tests Reveal That Not All Lycopene Supplements Contain What They Claim.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

CL Answer
What are the health benefits of amla and is it safe?
Learn about amla (Indian gooseberry), including its potential health benefits and possible safety concerns.

Product Review
Cinnamon Supplements and Spices Review
CAUTION: Some Cinnamon Products High in Toxin. See Which Passed or Failed and our Top Picks!

Product Review
Olive Leaf Extract Supplements Review
Learn What Olive Leaf Does and the Best Brands

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

CL Answer
What is Marine-D3 with Seanol-P, and does it live up to anti-aging claims?
Learn about the evidence for Marine-D3, a supplement from Marine Essentials that contains Seanol, including evidence from clinical studies on aging.

CL Answer
Can CBD cause a drop in blood pressure?
Find out if CBD (cannabidiol) can lower blood pressure or affect heart rate, plus information about other CBD side effects, drug interactions and safety concerns. ConsumerLab.com's answer explains.
Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

Clinical Update
12/13/2016
Cocoa for High Blood Pressure?
A new study in which people with mild hypertension were given a cocoa beverage and dark chocolate daily for eight weeks found that it only helped lower blood pressure when people were taking certain blood pressure-lowering medications. For more details about this and other studies with cocoa and dark chocolate, as well as our tests and comparisons of products, see the "What It Does" section of the Cocoa Powders, Dark Chocolate, Extracts, Nibs & Supplements Review >>
Clinical Update
8/02/2022
Hawthorn for High Blood Pressure?
Can taking hawthorn lower blood pressure in people with hypertension? Find out what research shows in our updated answer to the question: Which supplements can help to lower blood pressure?
Clinical Update
10/09/2018
Can a B Vitamin Lower High Blood Pressure?
A common mutation of the MTHFR gene is associated with an increased risk of hypertension. A recent study tested whether supplementing with a specific B vitamin could reduce blood pressure in affected individuals. Get the results in the MTHFR section of the B Vitamin Supplements Review. (Also see our Top Picks among B vitamins.)
Product Review
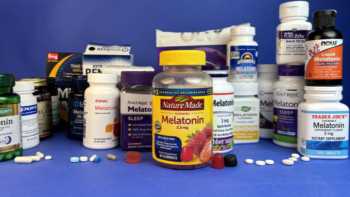
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
Product Review
Resveratrol Supplements Review (From Red Wine, Knotweed, and Other Sources)
See Which Resveratrol Supplements Were Best In Our Tests and Comparisons. Learn What Resveratrol Can and Can't Do.

Product Review
Iron Supplements Review (Iron Pills, Liquids and Chews)
See Which Iron Supplements Are CL's Top Picks for Different Needs

CL Answer
Do "lite" salts and salt substitutes containing potassium help lower blood pressure and reduce heart risks? Are they safe?
Find out if replacing regular salt with "lite" salt or salt substitutes, such as Morton Lite Salt, Morton Salt Substitute, NoSalt Original Sodium Free Salt Alternative, and MySalt, helps to reduce blood pressure, or decrease the risk of heart attack and stroke. Plus, learn about the safety and side effects associated with salt substitutes, drug interactions, and more.

Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

CL Answer
Does Rooibos Tea Have Health Benefits and Is It Safe?
Learn about the health benefits of rooibos tea, including red and green, unfermented rooibos tea, how is differs from black and green tea, safety, and potential drug interactions with rooibos tea.

Product Review
Sexual Enhancer Supplements Review (with Yohimbe, Horny Goat Weed, Arginine)
Choose the Best Sexual Enhancer Supplement. Only 30% of Selected Sexual Enhancement Supplements Pass Quality Tests.

Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

Clinical Update
8/18/2013
Vitamin D for Hypertension?
Some studies have suggested a modest reduction in high blood pressure with vitamin D supplementation. However, a recent study did not find such a benefit - although a closer look reveals why these results may not tell the full story. Get the details, plus more information about vitamin D and cardiovascular health, and test results for 55 vitamin D supplements, in the updated Vitamin D Supplements Review >>
Product Review
CBD Oils, Softgels, Gummies, Creams & Salves Review
See How Much CBD and THC We Found in Products.

CL Answer
Can fisetin, also called Cognisetin and Novusetin, really improve memory?
Fisetin (Cognisetin and Novusetin) information, including evidence from clinical studies on memory improvement, brain health, dosage, and pricing.

CL Answer
What is hesperidin, what is it used for and is it safe?
Learn about hesperidin, including whether its beneficial for heart health, problems with circulation, stroke, cognitive function, depression, cancer and other conditions, and find out if it is safe.

Product Review
Berberine and Goldenseal Supplements Review
Tests Reveal the Best and Worst Berberine and Goldenseal Products

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

CL Answer
What is Noopept? Can it really improve memory and cognition, and is it safe?
Learn more about Noopept, including results from clinical studies on cognition, safety, and dosage.
CL Answer
What are the benefits of grape seed extract?
Learn about the health benefits of grape seed extract, and find out it improves venous insufficiency, leg swelling, swelling after surgery, or can help to lower blood pressure.

CL Answer
Can prenatal vitamins have too much folic acid? Mine has 800 mcg, but isn't that more than what's recommended? Is this dangerous to me or my baby?
Is it possible for prenatal vitamins to have too much folic acid? Find out the correct dosage so that it is not dangerous to babies.

CL Answer
Which supplements reduce the risk of stroke? Which increase the risk of stroke?
Find out which supplements (may help reduce the risk of stroke/might increase the risk of stroke.)

Clinical Update
7/29/2022
Cocoa Flavanols & Blood Pressure
Do the flavanols in cocoa and dark chocolate lower blood pressure? See what a recent study found in people with normal blood pressure, as well as results for people with hypertension.
CL Answer
Are There Supplements That Help Treat POTS (Postural Orthostatic Tachycardia Syndrome)?
Find out which supplements and lifestyle changes may be helpful for people with Postural Orthostatic Tachycardia Syndrome (POTS).

CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
CL Answer
How to Reduce Lightheadedness and Dizziness When Standing Up (Orthostatic Hypotension)
Learn about movements that can reduce lightheadedness and dizziness that can occur from a drop in blood pressure upon standing (initial orthostatic hypotension). Plus find out if supplements such as vitamin D and CoQ10 may help.

CL Answer
Which oils can help lower my cholesterol and risk of heart attack? How are coconut oil, olive oil, and fish oil for example?
Find out which fats and oils, such as coconut oil and fish oil, lower cholesterol and lower the risk of cardiovascular disease.

CL Answer
Could taking vitamin C (500 mg), CoQ10, grape seed extract, fish oil, vitamin D3/calcium/magnesium/zinc interfere with taking beta-sitosterol with dinner?
Learn about drug interactions & proper dosages for supplements such as CoQ10, vitamin D & C, fish oil, magnesium, zinc and grape seed extract.

Product Review
Vitamin A Supplements Review, Including Beta-Carotene and Cod Liver Oil
Find the Best Vitamin A Supplement. Best Quality Vitamin A Supplements Identified, Including Cod Liver Oil.

Clinical Update
3/10/2021
Coconut Oil for Lowering Blood Pressure?
Can coconut oil lower blood pressure in people with mild hypertension? See what a new study found in the What It Does section of our Coconut Oil and MCT Oil Review. Also see our Top Picks among coconut and MCT oils.
CL Answer
Does coconut water help re-hydrate as well as sports drinks?
Find out how coconut water compares to sports drinks, including rehydration and possible side effects.

CL Answer
How do I find the best quality olive oil and what are the health benefits?
Health Benefits of olive oil, including heart benefits, anti-inflammatory effects. Plus, how to choose a quality olive oil and cook with olive oil

CL Answer
How much magnesium do I need and how much magnesium from supplements is too much?
Find out how much magnesium you should be getting on a daily basis, signs of magnesium deficiency, and how much magnesium is too much, particularly from magnesium supplements and laxatives containing magnesium. Find out the side effects and potential adverse effects of too much magnesium.

Product Review
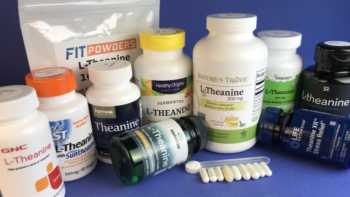
L-Theanine Supplements Review
Touted for Stress Relief, Do L-Theanine Supplements Work?
CL Answer
Can taking CoQ10 affect my thyroid levels or interact with my thyroid medication?
Learn more about CoQ10 and thyroid medications along with natural thyroid function. ConsumerLab.com's answer explains.
Product Review
Black Seed Oil Review
Find Out Which Black Seed Oil Supplements Passed or Failed Our Tests. See Our Top Picks.

CL Answer
Are there any supplements I should avoid when taking acetaminophen (Tylenol)?
Find out if there are any supplements that should be avoided when taking acetaminophen (Tylenol).
Product Review
Noni Juice and Supplements Review Article
What Are the Benefits of Noni Juice? Get the Facts About Noni Juice and Supplements.

Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

CL Answer
Is it better to use a powdered product that you mix with liquid than to take a pill? I was told that the vitamins will be more completely absorbed. Is that true?
Learn more about taking supplements & multivitamins in powder or tablet form, including tips on how to get the most from your supplement.

CL Answer
Risks of Too Many Vitamins & Supplements
Taking too many vitamin supplements -- or taking too much of a particular supplement -- can have short and long-term adverse effects.

CL Answer
What supplements should I stop taking before surgery?
Info on which supplements to avoid before surgery due to increased risk of bleeding or interference with anesthesia.
CL Answer
I'm worried that I'm not getting enough potassium, but potassium supplements only provide a small dose -- much less than the daily requirement. Why is this, and how can increase my daily intake?
Find out why many potassium supplements contain small doses of potassium and how to get adequate potassium from food and supplements.
CL Answer
Does Carditone work to lower blood pressure?
Carditone information, including safety and side effects, whether Carditone has been shown to work for blood pressure and angina, and more.
CL Answer
Should you take olive oil as a supplement?
Although extra virgin olive oil has many possible health benefits, such as reduced risk of heart disease and improved blood sugar control, these and other benefits have been demonstrated when olive oil replaces for saturated fats in the diet, not when taken as a supplement, as we explain.

CL Answer
Is it true that eating Brazil nuts can help with weight loss? Are there other health benefits or risks of Brazil nuts
Learn more about vitamin E intake from nuts, including Brazil nuts, hazelnuts, and almonds.

CL Answer
Do any supplements help for varicose veins or chronic venous insufficiency?
Find out which supplements help for varicose veins and chronic venous insufficiency, including citrus bioflavonoids and grape seed extract.

CL Answer
I have been having dizziness for the past few months and am wondering if it could be a side effect of supplements I take. Which supplements cause dizziness?
Find out which supplements that may cause dizziness, vertigo, or imbalance, including garlic, melatonin, saw palmetto, and red yeast rice.

CL Answer
Which supplements or foods can help lower cholesterol and keep my heart healthy? Are there any to avoid?
Information about heart health supplements & vitamins that help with cholesterol, including sterol esters, CoQ10, and vitamin D, and which may be bad for the heart, like calcium.

CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

CL Answer
Is it better to take fish oil, flaxseed oil -- or both?
Fish oil and flaxseed oil -- how they are different, what they are used for, if and how they can help with arthritis, depression, lowering cholesterol & more.

CL Answer
Can vitamins or other supplements cause a change in the ability to taste, or even a loss of taste?
Find out if supplements or vitamin deficiencies can cause changes in taste, loss of taste or smell. Information about vitamin D, zinc, vitamins A, B6, B12 and others. Plus medications and medical conditions that can alter taste and smell. ConsumerLab.com's answer explains.

CL Answer
Are L-carnosine supplements helpful and safe?
Learn about the potential health benefits of L-carnosine, as well as possible safety concerns, drug interactions and cost.

CL Answer
Is canola oil good or bad for you? I heard it can be toxic.
Find out if canola oil is bad for you or good for you, and whether it helps heart health. Clinical evidence and how it compares to other oils.

CL Answer
What is graviola? Can it really help fight cancer?
Learn more about graviola, if it can really help fight cancer, and its safety.

CL Answer
What are the health effects of apigenin and is it safe?
Apigenin, a flavonoid compound, is promoted for sleep, depression, cancer, and other conditions. Find out if it works and if it's safe.

Clinical Update
6/02/2013
Glucosamine May Increase Eye Pressure
New research suggests that people with glaucoma or intraocular hypertension could have worsening eye pressure if they take a glucosamine supplement. Get the details (along with our tests of glucosamine and other joint health supplements) in the updated Glucosamine/Joint Health Supplements Review >>
Clinical Update
5/28/2019
Glucosamine & Eye Pressure
Glucosamine may increase eye pressure in people with glaucoma or intraocular hypertension. But does it have this effect in people who don't have these conditions? See what a new study found in the Concerns and Cautions section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine and chondroitin.
Clinical Update
11/06/2018
Flaxseed Oil for High Blood Pressure?
Does flaxseed oil (which is rich in the omega-3 fatty acid ALA) help lower blood pressure in people with high blood pressure? Learn what a recent study found in the What It Does section of the Flaxseed Oil Review. (Also see our Top Picks for flaxseed and other seed oils.)
Clinical Update
9/09/2024
Potassium for High Blood Pressure?
How much potassium intake is recommended if you have high blood pressure and how should you get it? Find out in the Potassium Supplements Review, which includes our Top Picks for potassium as well as good options for potassium-enriched salts.
Also see: Which supplements can help to lower blood pressure?
CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

CL Answer
What are the health benefits of tart cherry juice?
See the evidence for tart cherry juice health benefits from clinical studies. Find out if tart cherry has anti-inflammatory effects, if it can improve sleep, lower high blood pressure, or help for muscle pain and osteoarthritis.

Clinical Update
6/24/2014
Vitamin D May Help Lower Blood Pressure
A recent study in people with mild to moderate high blood pressure showed that taking a vitamin D supplement helped lower blood pressure. Subjects in the study were already taking a calcium channel blocker (nifedipine), but taking vitamin D reduced blood pressure by several additional points. For details, including the dose of vitamin D used, as well as our tests of vitamin D supplements which can provide this dose, see the updated Vitamin D Supplements Review (Including Calcium, Vitamin K, and Magnesium) >>
CL Answer
Which supplements and foods can help lower or control blood sugar?
Find out which supplements can help lower or control blood sugar. Supplements including turmeric/curcumin, fiber, cinnamon, and ginseng are believed to help lower blood sugar.
Clinical Update
3/21/2015
Vitamin D for Blood Pressure?
Low levels of vitamin D in the blood are associated with high blood pressure. However, a recent review of many studies concludes that giving vitamin D doesn't help lower blood pressure. For more details about this and other potential uses of vitamin D, as well as our test-based reviews of products, see the Vitamin D Supplements Review >>
CL Answer
Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Find out if natural remedies & supplements for coronavirus such as zinc, vitamin C, garlic, or elderberry help to prevent or treat COVID-19.
CL Answer
Rhodiola and ashwagandha are both promoted to help with stress and anxiety. Is it safe to take them at the same time?
Find out if it is safe to take ashwagandha and rhodiola together. Both herbs are promoted to help with stress and anxiety, but may also lower blood pressure and have other side effects. ConsumerLab.com's answer explains.

Clinical Update
3/28/2014
Fish Oil Lowers Blood Pressure
Fatty acids from fish and fish oil may reduce blood pressure, particularly in those with high blood pressure, according to a recently published review. For details, information about other uses of fish oil, and our tests of fish oil supplements, see the updated Fish Oil and Omega-3 Fatty Acid Supplements Review >>
Clinical Update
5/20/2012
B Vitamin May Lower Blood Pressure
According to a new study, approximately 10% of the population is genetically prone to high blood pressure which may respond dramatically to a low dose of riboflavin (vitamin B-2). Read the update to the B Vitamin Supplements Review for details, including the dose used and amount of blood pressure reduction achieved. More >>
Clinical Update
11/24/2018
Yogurt & High Blood Pressure
Higher consumption of yogurt was associated with a lower risk of developing high blood pressure in a recent analysis. Risk was further reduced when yogurt consumption was part of a particular, healthful diet. For details, see the Fermented Dairy Foods section of the Probiotic Supplements Review.
Clinical Update
5/15/2020
High Blood Pressure Risk With Too Much Folic Acid During Pregnancy
While it is important to take folate during pregnancy, a new study suggests that too much folic acid from supplements increases the risk of high blood pressure in mothers. See the details in the Folic Acid section of the B Vitamin Supplements Review.
Also see our Top Pick among prenatal vitamins, which provides adequate (but not too much) folic acid, in our Multivitamin and Multiminerals Supplements Review.
Clinical Update
8/21/2020
Beetroot for High Blood Pressure?
Beetroot juice can lower blood pressure – but by how much? Learn what a recent study showed in our updated answer to the question: Can beetroot juice or supplements help lower my blood pressure?
Clinical Update
8/12/2024
High Blood Pressure from "Liver Support" Supplement
A woman developed high blood pressure after daily use of a “liver support” supplement. Find out the likely reason why in our article about licorice root tea and supplements.
Clinical Update
12/27/2022
Coffee and High Blood Pressure
Even modest amounts of coffee may increase risk of heart attack or cardiovascular-related death in people with very high blood pressure, according to recent study. Get the details in our updated article about coffee and heart health.
Also see this recent update: Coffee's Effects on Vitamins & Minerals
CL Answer
My HDL (good) cholesterol level is low. Should I take niacin to raise my HDLs?
Learn more about niacin and find out if it can raise HDL ("good") cholesterol levels.

CL Answer
I've heard that grapefruit juice can interact with medications because it inhibits an enzyme that breaks down drugs in the body. Do any supplements interact the same way with drugs?
CYP3A4 inhibitors, such as grapefruit, can interact with certain medications by inhibiting the liver enzyme that metabolizes many drugs. ConsumerLab.com's answer explains.

CL Answer
I take warfarin (Coumadin), an anticoagulant drug. Are there supplements I should avoid, or be taking, due to this drug?
Find out which supplements should be avoided when taking blood thinners (anticoagulants) like warfarin (Coumadin), including vitamin K, St. John's wort, and curcumin.

CL Answer
What are the health benefits of serrapeptase?
Serrapeptase is a proteolytic enzyme. Learn more about its health effects, including whether evidence supports its use for pain, inflammation, and heart health.

CL Answer
10 Supplements That May Boost Testosterone (And 13 That May Not)
Many supplements promise to increase testestorone, boost libido, and increase muscle size, but, as ConsumerLab explains, the actual effectiveness of supplements is limited, and some supplements may actually reduce testosterone levels.
Clinical Update
1/10/2021
Magnesium, Niacin and Blood Pressure
Two studies this week showed the risk of high blood pressure to be lowest among people with adequate, but not excessive, dietary intakes of magnesium and niacin.
Also, see which foods are good sources of magnesium and, separately, good sources of niacin.
Clinical Update
3/30/2019
Green Tea for Lowering Blood Pressure
Drinking green tea can help lower high blood pressure — and even reverse ventricular hypertrophy, according to a new study. For details, including the amount and brand of tea used, see the What It Does section of the Green Tea Review. Also see our Top Picks for green tea.
Clinical Update
2/16/2016
Fish Oil and Blood Pressure
As explained in our Fish Oil Review, fish oil supplements may not provide overall cardiovascular benefits — eating fish is better. However, a recent analysis found that a certain kind of fish oil may help people with high systolic blood pressure. For details, see the Fish Oil and Omega-3 Fatty Acids Supplements Review >>
Clinical Update
1/23/2019
Garlic and Blood Pressure
If you have high blood pressure, a particular garlic supplement may provide some benefit, according to a recent study. For details, see the "What It Does" section of the Garlic Supplements Review. Also see our top choices for garlic supplements.
Clinical Update
7/26/2014
Probiotics Lower Blood Pressure
Probiotics may help lower high blood pressure, especially when multiple strains are taken for a certain period of time, according to a new analysis. Get the details, as well as information about other uses for probiotics, in the updated Probiotic Supplements Review >>
CL Answer
What are the side effects of magnesium supplements?
Info on magnesium supplement side effects, such as stomach upset, nausea and diarrhea. Learn about magnesium dosage, safety and drug interactions. ConsumerLab.com answer explains.
Clinical Update
6/09/2018
Magnesium & Metabolic Syndrome
A study shows that magnesium can significantly help some people suffering from metabolic syndrome (i.e., high blood sugar, high blood pressure, and related symptoms). Get the details in the What It Does section of the Magnesium Supplements Review. Also see our Top Picks among magnesium supplements.
CL Answer
How does Athletic Greens’ AG1 compare to multivitamins and Greens powders? Is AG1 worth it?
ConsumerLab reviews the ingredients in AG1, its safety and possible side effects, and how AG1 compares to other products.

CL Answer
Which vitamins and supplements should be stopped before getting blood work, laboratory tests, or medical imaging?
Find out which vitamins and supplements can interfere with blood, urine or stool tests, and imaginging tests, like bone scans and MRIs. Learn how B vitamins such as biotin, niacin and riboflavin, as well as calcium supplements, St. John’s wort, vitamin C, and others supplements can affect test results, as well as common foods that may interfere with tests.
CL Answer
Do any supplements or foods help with migraine? Do any trigger migraine?
Find out if supplements such as magnesium, CoQ10, riboflavin, butterbur, feverfew and others help with migraine. Also learn about foods that may trigger migraine, including chocolate and watermelon.

CL Answer
Is it safe to consume fish oil as a long-term food supplement?
Learn more about fish oil supplement safety including long-term use, side effects, and more.

CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

CL Answer
When taking a statin drug like Lipitor or Crestor, are there supplements I should avoid or take?
Learn about the interactions between certain supplements and atorvastatin (Lipitor), rosuvastatin (Crestor), and other cholesterol-lowering statins.
CL Answer
Do any supplements help with fatty liver disease? Are some diets more beneficial than others?
Find out if supplements such as vitamin E, fish oil, milk thistle, curcumin, choline, probiotics, vitamin D, reishi mushroom, or extra virgin olive oil are beneficial for fatty liver disease and learn which diet (Mediterranean, ketogenic, DASH, or fasting diets) seems to have greatest benefit.
CL Answer
Which supplements and foods help with gout and which may worsen it?
Find out about the effects on uric acid levels and gout of fish and fish oil, coffee, beer, animal meat, milk proteins, vegetable proteins, fructose, lemon juice, tart cherry juice, vitamin A, vitamin C, and niacin
Clinical Update
6/30/2013
Vitamin D & Depression
Women with depression and type 2 diabetes were given high-dose vitamin D for several weeks in a small, pilot study. By the end of the study, vitamin D levels in the blood had risen considerably and mood was significantly improved, as was blood pressure. Although just a pilot study, this is not the first study to suggest that vitamin D may help with depression. For more details, as well as test results and comparisons for 55 vitamin D supplements, see the updated Vitamin D Supplements Review >>
CL Answer
Can taking too much fish oil be dangerous?
Find out if too much fish oil can be dangerous, and if taking too much fish oil can cause effects such as suppressing the immune system, thinning the blood (increasing the risk of bleeding), increasing levels of LDL cholesterol and liver enzymes or increasing the frequency in episodes atrial fibrillation. ConsumerLab.com's answer explains.

CL Answer
How do I choose the best magnesium supplement?
Choosing the best form of magnesium supplements can be tricky due to the many different types (magnesium aspartate, bicarbonate, carbonate, chloride, citrate and others).

CL Answer
What is C15:0 fatty acid (found in fatty15), and does it have health benefits?
C15:0 fatty acid in fatty15 is promoted for “healthier hair & skin, balanced metabolism, and deeper sleep,” as well as “slowing the aging process.” Find out if it works.

CL Answer
L-Tyrosine: Health Effects and Safety
Tyrosine has been evaluated for conditions including physical and mental stress, phenylketonuria (PKU), depression, and attention deficit-hyperactivity disorder (ADHD). Find out if it works and if it's safe.

CL Answer
Which is the best pulse oximeter for home use?
Best pulse oximeter for home use (COVID-19, asthma and COPD). Differences between home use and FDA-approved medical pulse oximeters.

CL Answer
Which supplements can help treat anemia? Do any supplements cause a low red blood cell count?
Find out which vitamin and nutrient supplements can help prevent and treat anemia in people who are nutrient deficient and learn which supplements may cause low blood cell counts, especially when used in excess.

CL Answer
Just one dose of a horse chestnut extract (standardized to 20% aescin) caused me to have significant nausea and vomiting. Might there be a problem with the product?
Find out if horse chestnut extract side effects, including gastrointestinal discomfort such as nausea and vomiting, and other safety information about horse chestnut.

CL Answer
What are the side effects of glucosamine and chondroitin?
Learn the side effects of glucosamine and chondroitin supplements for joint health and joint pain, including stomach upset, headache, and many others. ConsumerLab.com's answer explains.

CL Answer
Is it safe to take arginine, or other supplements for sexual enhancement, while taking Viagra or Cialis?
When taking Cialis or Viagra, is it safe to take arginine or other sexual enhancement supplements?

CL Answer
Does glucosamine raise "bad" LDL cholesterol?
Learn more about the link between glucosamine and cholesterol, including evidence from clinical studies.

CL Answer
Is it true that some vitamins or supplements can cause cancer?
Learn more about the effects of certain vitamins and supplements on cancer, including beta-carotene, vitamins E and B9, and multivitamins.

Clinical Update
9/18/2020
Vitamin D for Failing Hearts?
Does high-dose vitamin D help or hurt people with chronic heart failure? Find out what a new study showed in the Cardiovascular Disease section of Vitamin D Supplements Review, which also discusses cholesterol, blood pressure and heart attack risk. Also see our Top Picks for vitamin D.
Clinical Update
7/26/2022
Vitamin D and Heart Failure
Does high-dose vitamin D improve heart function in people with chronic heart failure? Find out what a recent study showed in the Cardiovascular disease, blood pressure, and cholesterol section of our Vitamin D Supplements Review.
Clinical Update
2/21/2024
Big Benefits of Salt Substitute
A study among older people with high blood pressure showed that use of a salt substitute led to a dramatic reduction in heart attack and stroke. Get the details in the Salt Substitutes section of our Potassium Supplements Review, which includes information about popular salt substitutes on the market.
CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

CL Answer
Does Gynostemma pentaphyllum really work as an AMPK activator and help for diabetes, high cholesterol, or weight loss?
Find out if Gynostemma pentaphyllum ( jiaogulan) works as an AMPK activator and helps to lower blood sugar and treat diabetes. ConsumerLab.com's answer explains.

CL Answer
A blood test showed my vitamin D level is low. How do I know how much vitamin D to take?
Find out how to increase vitamin D blood levels, including a good target range.

Clinical Update
4/01/2012
Vitamin D and the Risk of Death
In a six-year study of over 10,000 people in Kansas, the risk of death was found to be 164% higher among those with lower levels of vitamin D in their blood. These people were also more likely to suffer from hypertension, coronary artery disease, cardiomyopathy, and diabetes than those with higher levels. Only 29% of the people in the study had the higher level of vitamin D. For details, including the level of vitamin D associated with lower risk of death, ways to boost vitamin D, tests of vitamin D supplements, and more, see the Vitamin D Review >>
CL Answer
How Should You Take Care of Yourself After You’ve Had COVID-19?
Find out the best way to care for yourself when recovering from COVID-19, including how long you should quarantine, whether you should get vaccinated, when it is safe to undergo a surgery, and how to spot and, possibly, improve lingering symptoms.

CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

CL Answer
I'm considering taking vitamin K for my bones, but I take blood thinner (anticoagulant) medication. Is there a problem taking both?
Learn more about vitamin K, including info on interactions with blood thinners such as Coumadin and Jantoven, its effects on bones & impact on clotting.

CL Answer
Best Supplements to Prevent Age-Related Muscle Loss
Discover the top supplements to prevent age-related muscle loss after 60. Learn how protein, fish oil, vitamin D, and more can help maintain muscle strength and combat sarcopenia.

CL Answer
Is it possible to take too much vitamin C?
Find out the daily value of vitamin C, along with possible side effects of excessive vitamin C intake. ConsumerLab explores the topic of proper vitamin C supplement usage, and why taking too much can be harmful.
CL Answer
What is lumbrokinase, does it have heart benefits, is it safe, and what should you look for, or avoid, in a lumbrokinase supplement?
Lumbrokinase is marketed as promoting healthy circulation and supporting heart health. Find out how and if lumbrokinase supplements actually work, possible safety concerns, and what to consider when choosing a lumbrokinase supplement.

CL Answer
Is it possible to get too much vitamin D?
Too much vitamin D can lead to adverse effects, including hypercalcemia (excess calcium in the blood).

CL Answer
What are sugar alcohols and why are they in my nutrition bar?
Learn more about sugar alcohols, including maltitol and lactitol, why some products contain them, and possible side effects.

CL Answer
What are the side effects of ashwagandha supplements?
Learn about the safety and side effects of ashwagandha supplements, including headache, sleepiness, upset stomach, blood pressure and more. ConsumerLab.com's answer explains.

CL Answer
Is drinking coffee good or bad for heart health?
Learn more about coffee and caffeine, its safety, if it's good for you, how much coffee is too much and its impact on your heart.

CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

Clinical Update
5/10/2024
Supplements and Blood Pressure
Find out which supplements may increase or decrease blood pressure, and learn what to look for when selecting a home blood pressure monitor, in our article about supplements and blood pressure.
Clinical Update
9/27/2024
Size Matters with Blood Pressure Cuffs
The standard cuff that comes with some popular at-home blood pressure devices may cause an inaccurate reading for some people. Learn what a recent study of devices sold on Amazon showed in the Choosing a device to monitor blood pressure section of our article about supplements for lowering blood pressure.
Clinical Update
8/11/2023
Getting an Accurate Blood Pressure Reading
A new study shows why choosing the right-sized blood pressure cuff is critical for an accurate reading. Get the details in the blood pressure monitors section of our article about supplements and blood pressure.
CL Answer
What are trace minerals and do I need them?
Trace minerals include boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, zinc, and others. Find out what they do, how much of each trace mineral you should be getting, and who's most likely to be deficient in these minerals.
CL Answer
Which supplements help reduce the risk of colorectal cancer?
Find out which supplements may reduce the risk of colorectal cancer, including folate, fiber, selenium, and vitamin D.
CL Answer
Dietary Supplements and Herbs to Avoid When Breastfeeding
Find out which supplements should be avoided when breastfeeding, including medicinal herbs, soy, and flaxseed.

CL Answer
How can I avoid aconite – a toxin – in Aconitum supplements?
Aconite is a highly toxic alkaloid found in Aconitum plants ( also called monkshood, wolfsbane, or devil's hood). Aconite poisoning can cause low blood pressure, chest pain, increased or decreased heart rate, and irregular heart rhythm that can lead to cardiac arrest and death. Find out which supplements may contain aconite and how to avoid it.

CL Answer
10 Supplements That May Help Reduce Nighttime Urination (And 5 That May Not)
Waking up more than once during the night to use the bathroom? Learn more about the 9 supplements that may help and 5 others that don't.

CL Answer
What are the health benefits of dark chocolate?
Information on the health benefits of dark chocolate, including how dark chocolate may improve heart health, decrease blood sugar levels and more. ConsumerLab.com's answer explains.

CL Answer
I've heard that soaking dried beans for 24 hours reduces the phytate level, allowing for greater access to nutrients. Is this true?
Does soaking dried beans in water help reduce phytate levels to help gain greater access to nutrients?

CL Answer
Which supplements, foods or diet and lifestyle changes help relieve acid reflux (heartburn), and which worsen it?
Find out which supplements and foods can help relieve acid reflux, and which can make it worse.
CL Answer
What are the health benefits of manuka honey, and is it safe?
Manuka honey health benefits, how it differs from common, store-bought honey, safety, evidence for using manuka honey for wounds, sore throat and cough, constipation and more.

Clinical Update
4/30/2012
Vitamin C and Blood Pressure
Vitamin C supplements may modestly reduce blood pressure, particularly among people with elevated blood pressure, according to a review of the latest clinical studies. Get the details, as well as our tests of vitamin C supplements, in the updated Vitamin C Supplements Review. More >>
Clinical Update
8/28/2020
Acetyl-L-carnitine + Alpha-Lipoic Acid for Lowering Blood Pressure?
We were recently asked if taking a combination of acetyl-L-carnitine and alpha-lipoic acid can help lower blood pressure. Find out what research shows in the What It Does section of the Acetyl-L-Carnitine Supplements Review. Also see our answer to the question: Which supplements can help to lower blood pressure?
Clinical Update
7/22/2022
Fisetin & Blood Pressure
A CL member taking anti-hypertensive medication reported that a dose of fisetin reduced her blood pressure for several hours. Find out if this is a known effect in our updated Fisetin article.
Also see our CL Answer about supplements that may affect blood pressure.
Clinical Update
11/10/2023
Hibiscus for Blood Pressure?
Can supplementing with hibiscus flower in capsule or tea form help lower blood pressure? Find out in our article about supplements for lowering blood pressure.
Clinical Update
11/28/2023
L-Arginine & Blood Pressure
Find out why L-arginine should be used cautiously if you are taking medication that reduces blood pressure (including medications for ED) in the Concerns and Cautions section of our L-Arginine Supplements Review.
Learn about other supplements that can affect blood pressure.
Clinical Update
10/28/2022
Citrulline for Blood Pressure?
Does L-citrulline supplementation help lower blood pressure? Find out what a recent study showed in the Citrulline section of our L-arginine Supplements Review.
Also see our answer to the question: Which supplements can help to lower blood pressure?
Clinical Update
5/05/2023
Getting an Accurate Blood Pressure Monitor
Many of the best-selling blood pressure monitoring devices sold on Amazon in the U.S. and Canada aren’t validated for accuracy, according to a recent study. We’ve added information about this and how to find a validated device to our article about supplements that can affect blood pressure.
Clinical Update
2/09/2024
Coffee & Blood Pressure
Does drinking regular coffee increase blood pressure in people taking blood pressure-lowering medication? See what a recent study showed in our article about coffee and heart health.
CL Answer
Which foods and supplements contain lead, cadmium, or arsenic?
Discover which foods and dietary supplements contain lead and other metals, the limits, who’s testing, and how to stay safe.

CL Answer
Is there a danger in taking lecithin or phosphatidylcholine? I heard that they may increase the risk of heart attacks.
Lecithin, phosphatidylcholine and choline - Learn more about the potential link between them and cardiovascular disease.

CL Answer
Does TA-65 Astragalus Extract Increase Telomere Length and Longevity?
TA-65 Astragalus Extract is promoted to increase telomere length. Find out if there is evidence for this, if this increases lifespan or longevity, plus safety, side effects and cost.

CL Answer
Do hair loss supplements, such as Viviscal, Hair La Vie, and Nutrafol, or topical essential oils work?
Vitamins and supplements that may help with hair loss and thinning, including saw palmetto, beta-sitosterol, protein, iron, and vitamin D.

CL Answer
Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Find out which supplements for nerve pain, including fish oil and alpha-lipoic acid, are effective for sciatica, diabetic neuropathy, or postherpetic neuralgia.

CL Answer
I thought the B vitamins were all water soluble and did not build up in the body, so you would not build up toxic levels. Am I wrong?
Learn more about water-soluble B vitamins including niacin, B6, B12 and folic acid, and why taking too much of these vitamins can be toxic. See the symptoms of overdose and toxicity from B vitamins. ConsumerLab.com's answer explains.

Clinical Update
2/01/2022
Vaccines, Blood Pressure, and Thyroid Symptoms
Find out if COVID-19 vaccines increase blood pressure or worsen symptoms of thyroid conditions.
CL Answer
Which supplements can help lower my triglycerides?
Find out whether fish oil supplements, or plant-based oil supplements, such as flaxseed oil and echium oil are best for lowering triglyceride levels. ConsumerLab.com's answer explains.
CL Answer
Is sublingual vitamin B-12, which is placed under the tongue and allowed to dissolve, really better than the pill form?
Learn the difference between sublingual vitamin B-12, vitamin B-12 pills and more through information including clinical evidence.

CL Answer
I've been using a lutein supplement with 40 mg of lutein and am concerned that this dose may be too high. Are there known harmful side-effects?
Learn more about lutein side effects, proper dosage and safety in vision supplements.

CL Answer
Are there supplements I should avoid when taking apixaban (Eliquis) or similar blood thinner drugs like Xarelto?
Learn about supplement interactions with apixaban (Eliquis), rivaroxaban (Xarelto) and other blood thinner drugs in the direct factor Xa inhibitors class.
Clinical Update
12/09/2022
Blood Pressure Meds and Potassium
Be aware that many blood pressure-lowering medications can increase potassium in the blood to dangerous levels, as was recently reported. Get the details in the Concerns and Cautions section of our Potassium Supplements Review.
CL Answer
Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Find out which supplements help improve memory, brain function and cognition, including fish oil, some B vitamins, cocoa, and curcumin. ConsumerLab's answer explains the evidence for supplements promoted to help with brain function and cognition.
CL Answer
I'm over 50 and looking to take a vitamin B-12 supplement. Many contain a form of vitamin B-12 called cyanocobalamin, yet I read on the Internet that this form is toxic. Should I be concerned?
Looking to see if the cyanocobalamin form of vitamin B-12 is safe? Read research and safety information on ConsumerLab's report.

CL Answer
Does taking a laxative interfere with the absorption of vitamins or minerals?
Information on laxative interactions with other medications and supplements such as Maalox, antibiotics, and statins and diabetes medications.

CL Answer
Are there any serious side effects with Lovaza or Epanova (prescription fish oil)? If so, would these also apply to generic versions of these drugs and to fish oil supplements?
Understand the side effects of prescription fish oil like Lovaza and Epanova, and find out if the generic fish oils are effective and safe.

Clinical Update
7/06/2022
Whey Protein & Blood Pressure
Whey protein may temporarily lower blood pressure. Find out if adding carbohydrates and fats reduces this effect in the Concerns and Cautions section of our Protein Supplements Review.
Clinical Update
5/10/2022
Whey Protein & Blood Pressure
Older people may experience a dip in blood pressure after consuming whey protein, according to a recent study. Get the details in the Concerns and Cautions section of our Protein Powders, Shakes, and Drinks Review.
Clinical Update
2/14/2023
Hawthorn for Heart Health?
Can hawthorn berry supplements lower cholesterol and improve heart health? Find out in our updated CL Answer about cholesterol lowering supplements.
Also find out if hawthorn berry lowers blood pressure in our article about supplements for lowering blood pressure.
Clinical Update
4/14/2023
Oral Hygiene and Blood Pressure
Can oral hygiene practices lead to increased or decreased blood pressure? Find out what research suggests in our updated CL Answer about toothpastes and dental products.
Clinical Update
1/27/2024
Licorice & Blood Pressure
Eating black licorice, or drinking tea that contains it, can increase blood pressure and cause heart issues. Get the latest details in our article about licorice.
Clinical Update
1/09/2024
Ceramides and Blood Pressure?
Does supplementing with phytoceramides (“plant” ceramides), which are promoted for hydrating and plumping the skin, increase blood pressure? Find out in the Safety and Side Effects section of our article about phytoceramides.
Clinical Update
8/18/2023
Blood Pressure Meds & Hair
Be aware that different types of blood pressure medication have different effects on hair growth. Get the details in our article about hair growth.
Clinical Update
6/28/2024
Beetroot for Blood Pressure?
Did taking beetroot extract decrease blood pressure among healthy older men and women? See what a recent study found in our article about beetroot juice and supplements.
Also see how the nitrate content in popular beetroot products compare, and learn about potential side effects and interactions with beetroot.
Clinical Update
1/14/2022
Magnesium for Lowering Blood Pressure?
The FDA recently allowed supplements and foods that provide magnesium to make a claim regarding blood pressure reduction. But there is more to the story. Get the details in the What It Does section of the Magnesium Supplements Review. Also see our Top Picks among magnesium supplements.
Clinical Update
5/04/2021
Grape Seed Extract for Blood Pressure?
Can grape seed extract lower blood pressure? See what a new study found in our article about the Health Benefits of Grape Seed Extract.
Clinical Update
9/13/2020
Low Vitamin D and Low Blood Pressure?
Vitamin D deficiency has been linked with a form of low blood pressure (orthostatic hypotension) in older men. Get the details in the What It Does section of Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
9/22/2020
Magnesium for Lowering Blood Pressure?
Can magnesium supplementation lower elevated blood pressure? Find out what a new study showed in the What It Does section of the Magnesium Supplements Review. Also see our Top Picks for magnesium supplements.
Clinical Update
8/22/2014
Flaxseed Lowers Blood Pressure
Consuming milled flaxseed significantly lowered blood pressure in people with peripheral arterial disease, according to a recent clinical trial. Additional analysis of those results suggests the alpha-linolenic acid (ALA) -- an omega-3 fatty acid -- in flaxseed may be responsible for the effect. Get the details, including the amount of flaxseed used, plus our tests of flaxseed, evening primrose, borage, and other seed oils containing ALA and GLA, in the updated Flaxseed Oil, Evening Primrose Oil, Borage Oil, and Black Currant Oil Supplements Review >>
Clinical Update
7/13/2016
Magnesium Lowers Blood Pressure
Magnesium supplementation may help to lower blood pressure, but only in those who are deficient in the mineral, according to a new analysis. Get the details in the "What It Does" section of the Magnesium Supplements Review >>
Clinical Update
10/31/2017
CoQ10 for Orthostatic Hypotension
CoQ10 may help reduce the fall in blood pressure that occurs in orthostatic hypotension (low blood pressure upon standing) according to a recent report. See the details in the "What It Does" section of the CoQ10 and Ubiquinol Supplements Review >> [Also see our Top Picks for CoQ10 supplements in the Review.]
CL Answer
Dandelion Tea & Supplements: Health Benefits and Safety
Find out about the potential health benefits of dandelion tea and supplements, as well as safety, side effects, and potential drug interactions.

CL Answer
Which supplements may help with PCOS (polycystic ovarian syndrome)?
Many supplements are promoted to help treat symptoms of PCOS (polycystic ovarian syndrome), but do they really work? See the clinical evidence for inositol (myo-inositol and D-chiro-inositol), alpha lipoic acid, vitamin B-12, folic acid, vitamin D and more.

CL Answer
If I already take red yeast rice, would adding high-dose niacin help further reduce my cholesterol levels, and would this be safe?
Learn more about the effect of combining red yeast, statins and high-dose niacin on cholesterol and safety.

CL Answer
Are Hormone Harmony and other Happy Mammoth Supplements Worth Taking?
Are the supplements Hormone Harmony, Hormone Harmony Plus+, Prebiotic Collagen Protein, or Bloat Banisher by Happy Mammoth likely to be beneficial for menopause symptoms or digestive health? Find out.

CL Answer
Can taking too much vitamin B-6 be dangerous?
Find out if multivitamins contain excessive amounts of B-6, how much B-6 is too much, and symptoms of toxicity and overdose.

CL Answer
What are human growth hormone (HGH) supplements? What are the benefits? Are they safe?
Learn about the benefits and risks of human growth hormone (HGH) supplements and injections. Get science-based insights on usage and effects.
CL Answer
What is acrylamide? Is it true that coffee and cocoa contain this toxin?
Find out what acrylamide is, how much is found in coffee and cocoa, and how you can minimize your intake of acrylamide.

Recalls & Warnings
August 16, 2023
FDA Warns Hekma Center, LLC for Promoting Products to Treat Anemia, Diabetes, Depression, & More
On June 2, 2023, the FDA issued a warning letter to Hekma Center, LLC following review of the company’s website and social media, which found statements about the company’s Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa ...
Clinical Update
12/10/2021
Magnesium for Metabolic Syndrome?
Can magnesium supplementation lower blood sugar and blood pressure in people with metabolic syndrome? Learn what a new study found in the What It Does section of the Magnesium Supplements Review. Also see our Top Picks among magnesium supplements.
Clinical Update
10/20/2016
Vitamin D Sweet Spot
Having the right blood levels of vitamin D has many proven benefits, but too little and too much vitamin D can be problematic. A new study shows this also to be true in people with diabetes with regard to a type of nerve damage that can affect blood pressure and heart rate. For details see the "What It Does" section of the Vitamin D Supplements Review >>
Clinical Update
3/15/2015
Folic Acid Reduces Risk of Stroke
A large study in people taking blood pressure-lowering medication found that the addition of a daily folic acid (vitamin B-9 or folate) supplement significantly reduced the chance of having a stroke. This benefit is most likely to occur among people whose blood levels of folate are low. More details, including dosage and ConsumerLab.com's test-based quality ratings of folic acid supplements, are found in the B Vitamin Supplements Review >>
Clinical Update
9/03/2024
fatty15 For Overweight/Obesity?
Does taking the fatty acid pentadecanoic acid (aka C15:0 fatty acid, found in fatty15) reduce weight or improve blood pressure, cholesterol, or blood sugar levels among people who are overweight or obese? Find out what a recent study showed our article about C15:0 fatty acid.
Clinical Update
10/25/2022
Symptoms of B-12 Deficiency
Vitamin B-12 deficiency can cause a wide range of adverse effects affecting the nervous system, cognition, blood and blood pressure, and more. Learn about the symptoms and recent cases, and find out who is most likely to be deficient in our B Vitamin Supplements Review.
Also see our article: Top 8 Supplements for Vegetarians and Vegans.
Clinical Update
3/03/2025
Biotin Caution With Lab Tests
Taking high-dose biotin can cause blood tests to show falsely high levels of testosterone and/or cortisol, as shown in recent cases. It can interfere with many other tests, as well. Get the details, in the Biotin section of our B Vitamin Supplements Review. Also see our Top Picks for biotin.
Also see: Which vitamins and supplements should be stopped before getting blood work and laboratory tests?
Recalls & Warnings
March 01, 2023
Seller of Dr. Miller’s Herbal “Detox” Teas Warned by FDA for Drug Claims
On February 3, 2023, the FDA issued a warning letter to Jackson Health & Wellness Clinic after inspection of the company’s website found statements about the company’s Dr. Miller’s Holy Tea, Dr. Miller’s Super Holy Tea, Dr. Miller’s Ultimate Tea, Dr.
Clinical Update
2/28/2023
Beetroot Extract for Exercise?
Does supplementing with beetroot extract improve exercise? See what a recent study found in our updated CL Answer about beetroot for blood pressure and exercise.
Clinical Update
2/06/2024
Salt Substitutes
"Lite" salts and salt substitutes containing potassium can help to modestly lower blood pressure. What should you look for in a salt substitute and how do products compare? Find out in our Potassium Supplements Review, which also explains who should not use salt substitutes.
Clinical Update
7/28/2023
Toothpastes, Mouthwash, and Probiotics for Oral Health
We answered the following questions this week, all of which relate to our article about toothpastes and other dental products:
- Can hydroxyapatite toothpaste prevent cavities as effectively as fluoride toothpaste?
- Does chlorhexidine mouthwash affect blood pressure?
- Why do some toothpastes contain glycine, and is this ingredient beneficial?
- Are probiotics beneficial for oral health?
Also, find out the abrasiveness of two Fluoridex brand toothpastes.
Clinical Update
7/12/2022
Citrulline for Heart Health?
Does supplementing with citrulline reduce blood pressure and improve heart function in people with heart failure? Find out in the Citrulline section of our L-Arginine Supplements Review (citrulline is converted to L-arginine in the body).
Clinical Update
2/18/2022
Help for Dizziness When Standing
Many people experience lightheadedness when standing up due to a temporary drop in blood pressure but certain techniques may help, according to a new study. Get the details in our new article How to Reduce Lightheadedness and Dizziness When Standing Up (Orthostatic Hypotension).
Clinical Update
5/10/2022
Choosing a Tart Cherry Product
Which tart cherry products are best for reducing muscle pain, improving sleep, or lowering blood pressure or cholesterol? Although the evidence is not strong, we identified products with the most evidence behind them in our Tart Cherry article.
Clinical Update
1/30/2025
Beetroot Juice for Strength?
Does taking beetroot juice before exercise improve strength? Find out what a recent study showed in our article: Can beetroot juice or supplements lower blood pressure or improve exercise performance, and are they safe?
Clinical Update
4/10/2025
TA-65 for Vision or Heart Health?
Does TA-65 (astragalus root extract) help people with macular degeneration or reduce blood pressure, cholesterol, or inflammation? Find out in our updated article about TA-65.
Clinical Update
2/13/2024
Turmeric Instead of Salt
Adding certain spices, such as turmeric, can significantly reduce the amount of salt needed to add saltiness to food, according to a recent study. Get the details, and see our Top Pick among tested turmeric spices, in our Turmeric & Curcumin Review.
Also see: Do “lite” salts and salt substitutes containing potassium help lower blood pressure and reduce heart risks? Are they safe?
Clinical Update
3/12/2024
Low-Acid Coffee for Reflux?
Are “low-acid” coffees easier on the stomach or less likely to cause reflux than regular coffee? Find out now in our article about coffee.
Also find out how coffee affects:
Clinical Update
1/11/2015
More Cocoa Benefits
A recently published study showed that hot cocoa made with a flavanol-enriched powder resulted in improvements in cognitive performance as well as reductions in blood pressure and cholesterol, and improved glucose metabolism. The study was conducted in people ages 65 to 80 who drank the cocoa each morning. More details, as well as our tests of cocoa powders, are found in the Cocoa and Dark Chocolate Review >>
Clinical Update
1/18/2014
Green Tea "Blocks" Beta-Blocker
A new study shows that drinking green tea can drastically reduce the amount of a beta-blocker (nadolol, Corgard) absorbed into the bloodstream. Beta-blockers are typically taken to lower blood pressure, raising concern for people using green tea along with this beta-blocker and, possibly, related medications. Get the details, along with our tests of green tea drinks and supplements, in the update to the Green Tea Review >>
If you take a beta-blocker (which includes the popular drugs metoprolol (Lopressor, Toprol) and propranolol (Inderal)), be aware that chromium and CoQ10 may offer benefits.
Clinical Update
8/26/2020
Vitamin K & Heart Health
Can taking vitamin K supplements improve vascular stiffness or lower blood pressure in people with kidney disease? Find out what a recent study showed in the What It Does section of our Vitamin K Supplements Review. Also see our Top Picks for vitamin K.
Clinical Update
12/14/2019
B12 & the Vegetarian
Vegetarians and vegans are susceptible to vitamin B12 deficiency, which can be manifest by a range of health issues, including low blood pressure, as recently reported in an otherwise healthy young man. Learn more in the B12 section of the B Vitamin Supplements Review. Also see our Top Pick for vitamin B12.
Clinical Update
1/06/2018
Vitamin D's Cardiovascular Effect
Taking vitamin D can improve aspects of cardiovascular function in certain situations, as shown in a recently published study. Get the details in the "Cardiovascular disease, blood pressure, and cholesterol" section of the Vitamin D Supplements Review. (Also see our Top Picks among vitamin D supplements.)
Clinical Update
2/10/2021
Energy Drinks and Blood Pressure
Which people are more likely to have cardiovascular side effects from energy drinks? Find out what a new study showed in the Energy Drinks & Shots section of our B Vitamins Supplements Review.
Clinical Update
9/25/2020
Vitamin D for Dizziness When Rising?
Can vitamin D reduce symptoms of orthostatic intolerance (low blood pressure when standing up)? Find out what a new study in adolescents showed in the What It Does section of Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
6/01/2021
Vitamin D for Orthostatic Hypotension?
Can supplementing with vitamin D reduce the risk of orthostatic hypotension (low blood pressure when standing)? Find out what a recent study showed in the What It Does section of our Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
12/03/2021
Peanuts for Obesity?
Can eating peanuts help manage weight, or reduce blood pressure and cholesterol levels? Find out what a recent study found in our updated answer about Supplements and Weight Management.
News Release
January 09, 2025
Not All Black Seed Oil Supplements Contain What They Claim, According to ConsumerLab
White Plains, NY, January 9, 2025 — Black seed oil and extract supplements are promoted for controlling blood pressure, cholesterol, blood sugar, and arthritis pain, and more.
Clinical Update
8/25/2013
Antioxidants: Too Much of a Good Thing?
Clinical Update
2/02/2024
Lower Blood Sugar with Aloe?
Does drinking aloe juice right after a high-fat meal reduce spikes in blood sugar and blood fat levels? Find out what a recent study showed in the What It Does section of our Aloe Vera Supplements Review, which includes our Top Picks for aloe.
Clinical Update
2/28/2023
Kombucha for Blood Sugar?
Drinking kombucha (a fermented beverage containing probiotics) with a high-carb meal may blunt the rise in blood sugar after the meal, according to a recent study. Get the details in the What It Does section of our Probiotic Supplements Review, which includes our Top Picks among products.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
2/07/2023
Strontium and Blood Tests
Strontium is included in some "bone health" supplements, but be aware that very high doses of strontium may interfere with blood tests for calcium and bone density scans. Get the details in the Concerns and Cautions section of our Calcium and Bone Health Supplements Review, which includes our Top Picks among products.
Also see: Which vitamins and supplements should be stopped before getting blood work and laboratory tests?
Clinical Update
5/22/2018
Hair & Nail Supplements Can Skew Lab Tests
There are reports of high-dose biotin (common in "hair and nail" supplements) causing false readings on blood tests. Be sure to tell your doctor if you're taking high-dose biotin. Get the details in the Biotin section of the B Vitamin Supplements Review.
Clinical Update
7/17/2018
Lower Cancer Risk With High Vitamin D?
A recent analysis suggests that breast cancer risk is lowest with vitamin D blood levels at or above 60 ng/mL -- an unusually high level. Before you try this at home, be sure to read our commentary in the What It Does section of the Vitamin D Supplements Review. (Also see our Top Picks for vitamin D.)
Clinical Update
2/29/2020
Biotin May Skew Many Blood Tests
If you take a high-dose biotin supplement, be aware of the growing list of blood tests with which it may interfere. See the latest information in the Biotin section of the B Vitamin Supplements Review.
Clinical Update
2/12/2019
Vitamin C for Blood Sugar Control
High-dose vitamin C was found to help control blood sugar levels in people with type 2 diabetes in a recent study. For details, see the What It Does section of the Vitamin C Supplements Review. Also see our latest Top Picks for vitamin C.
Clinical Update
10/15/2019
Vitamin D & Blood Sugar Control
Maintaining moderate levels of vitamin D may aid blood sugar control, but, according to a recently published study, high-dose vitamin D supplementation may not help. For details, see the Diabetes, insulin resistance and glucose control section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
8/19/2015
Cocoa Increases Blood Flow to Brain
A small study found that drinking a cocoa drink high in flavanols significantly increased blood flow to the brain, which, the researchers propose, might have potential cognitive benefits. Get the details, including dosage, plus more about cocoa and memory, and our tests of popular products, in the Cocoa Supplements and Dark Chocolate Review >>
Clinical Update
10/06/2020
CBD Interaction With Warfarin
High doses of CBD may affect blood clotting and require a dosage adjustment in people taking the blood thinner warfarin, as recently reported. Get the details and learn about other interactions with CBD in the Concerns and Cautions section of our CBD and Hemp Extract Review.
Clinical Update
6/20/2023
Zinc Caution
Taking very high doses of zinc, even short-term, can cause dangerously low blood sugar in people with no previous history of low blood sugar, as recently highlighted in a series of reports. Get the details in the Concerns and Cautions section of our Zinc Supplements and Lozenges Review, which includes our Top Picks among zinc products.
Clinical Update
5/18/2021
Fish Oil Interaction With Medication
High-dose fish oil can significantly increase blood levels of certain immunosuppressants, according to new research. Get the details in the Concerns and Cautions section of our Fish Oil Supplements Review, which includes information about other drug interactions with fish oil.
Clinical Update
5/19/2013
Optimum Level of Vitamin D
New research shows that blood levels which are too low or too high for vitamin D are associated with increased risk of heart attack and earlier death. Find the "sweet spot" for vitamin D levels as well as test results for 55 vitamin D supplements in the updated Vitamin D Supplements Review >>
Clinical Update
5/09/2011
Did you hear that DHA (an omega-3 fatty acid in fish oil) might raise the risk of prostate cancer?
This was recently in the news. Higher levels of DHA in the blood were associated with increased risk of high-grade prostate cancer. However, the findings do not mean that you should stop taking fish oil or eating fish. We talked with the researcher of this study and learned more. Get the facts in our Fish Oil Supplements Review. More >>
Clinical Update
5/27/2015
Adding Vitamin D to Statin Treatment
A recent study among people taking statin medication for high cholesterol showed significant improvements in cholesterol levels when daily vitamin D was added as treatment. Improvements were greatest among those who began with lower blood levels of vitamin D. More details, along with our reviews of products similar to the one used in this study, are found in the Vitamin D Supplements Review >>
Clinical Update
1/10/2017
Potential Risk with High B-12
Among people who are malnourished, those with elevated vitamin B-12 levels in their blood were twice as likely to die when hospitalized as those with lower B-12 levels, according to a new study. The study authors suggest caution with inappropriate supplementation. For more details about the study, and vitamin B-12, see the B Vitamin Supplements Review >>
Clinical Update
4/28/2017
Vitamin D for Muscle and Balance?
Why did a recent study show that both high and low-dose vitamin D didn't improve muscle strength or balance in women despite the fact that other studies have shown vitamin D to help? The likely answer has to do with the vitamin D status (i.e., blood levels) of the people chosen to participate in these studies. To find out what you should do to improve your balance and reduce your chance of falling, see the "Muscle, Balance and Falls" section of the Vitamin D Supplements Review >>
Clinical Update
8/02/2016
Fish Oil Helps After Heart Attack
High-dose fish oil supplementation improved the heart's ability to pump blood in people recovering from a heart attack, a recent study found. Get the details in the "What It Does" section in our Fish Oil Supplements Review >>
Clinical Update
4/15/2015
Vitamin D and Statin-Related Muscle Pain
Taking high doses of vitamin D may reduce statin-related muscle pain in people with low blood levels of vitamin D, according to a new study. Get more information and our test reports on vitamin D supplements in the Vitamin D Supplements Review >>
Clinical Update
5/02/2015
Too Much Vitamin D
A U.S. study showed a recent 26-fold increase in the percentage of people with excessive blood levels of vitamin D, driven by increased supplement use. Although acute toxic effects were rare, potential long-term risks exist with these high levels. See the details in Vitamin D Supplements Review >>
Clinical Update
8/13/2019
Vitamin D for Breast Cancer?
Some evidence suggests that having higher blood levels of vitamin D lowers the risk of breast cancer. Can very high-dose vitamin D slow tumor growth in women with breast cancer? See what a new study found in the Cancer section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D supplements.
Clinical Update
9/01/2020
Folate & Memory
Older people with high blood levels of folate are more likely to perform worse on tests of memory and cognition unless they have sufficient levels of another B vitamin, according to a recent study. Get the details in the What It Does section of our B Vitamin Supplements Review. Also see our Top Picks among B vitamin supplements.
Clinical Update
12/05/2017
Biotin Interference With Vitamin D Test
Last week we reported that high doses of biotin (found in B complexes and hair and nail supplements) can interfere with certain blood tests. A study recently showed that biotin can also artificially inflate a test for vitamin D. Get the details in the "Biotin" section of the B Vitamin Supplements Review >>
Clinical Update
12/08/2016
Vitamin D Keeping You Up?
A study of women given daily doses of vitamin D found that those who achieved the highest blood levels of vitamin D experienced a deterioration in the quality of their sleep. This is consistent with an earlier study showing decreased production of melatonin with high-dose vitamin D. For details, see the "Concerns and Cautions" section of the Vitamin D Supplements Review >>
Clinical Update
2/04/2022
Risk of Too Much B-12
It’s easy to get way more vitamin B-12 than one needs from supplements. Unfortunately, high blood levels of B-12 are being linked to higher risks of death and disease, as shown in a recent U.S. study. Get the details in the Upper Limit for B-12 section of our B Vitamin Supplements Review. Also, see our Top Pick among B-12 supplements.
Clinical Update
2/04/2022
Vitamin D & Death From COVID
Some research suggests that low blood levels of vitamin D increase the risk of death from COVID, but a new study suggests that high levels also increase risk. Get the details in the COVID section of our Vitamin D Supplements Review.
Also see our answer to the question: Do any supplements help with the coronavirus (COVID-19)?
Clinical Update
6/02/2023
Biotin Caution With Lab & At-Home Tests
High doses of biotin from supplements can interfere with a wide range of laboratory and at-home tests, including those for pregnancy, doping, COVID, and heart attacks. Get the details in the Biotin section of our B Vitamin Supplements Review.
Also see: Which vitamins and supplements should be stopped before getting blood work and laboratory tests?
Clinical Update
6/06/2023
Caution With Calcium and Antacids
Getting too much calcium from supplements and/or over-the-counter antacids can cause dangerously high blood levels of calcium, as highlighted in a recent report. Get the details in the Concerns and Cautions section of our Calcium Supplements Review.
Also find out how much calcium is enough, and how much is too much, based on age and gender, in the ConsumerTips section of the Review.
Clinical Update
7/07/2023
Olive Oil & Inflammation
Does high-polyphenol extra virgin olive oil decrease inflammation compared to regular olive oil? See what a recent study found in our Extra Virgin Olive Oil Review.
Also see our Top Picks among extra virgin olive oils and learn what impact these oils may have on insulin and blood sugar control.
Clinical Update
11/04/2024
Try Taking Cinnamon This Way
A recent study suggested that taking cinnamon as a powder may be better for controlling blood sugar than taking it as a capsule. For details, see our Cinnamon Supplements and Spices Review, which includes our Top Picks among cinnamon supplements and spices.
Also be aware that the FDA recently reported high levels of lead in yet another cinnamon powder.
Clinical Update
1/06/2025
Risk With Too Much Vitamin A
Hypercalcemia (too much calcium in the blood) is a known risk when taking too much vitamin D, but it can also occur with high doses of vitamin A, as highlighted by a recent report. Get the details in our Vitamin A Supplements Review, which includes our Top Picks for vitamin A and beta-carotene.
Recalls & Warnings
May 24, 2023
Seller of Echinacea, High Blood Pressure Products & More Warned for Drug Claims
On May 12, 2023, the FDA issued a warning letter to H.E.A.L.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
July 15, 2024
Hard Steel Supplements for Men Recalled
On July 12, 2024, Supercore Products Group issued a recall of Hard Steel Capsules and Gold Hard Steel Liquid which are used for male erectile dysfunction. The FDA analysis found the capsules to contain sildenafil and acetaminophen.
News Release
December 05, 2024
Some Oats Surprisingly High in Gluten, According to ConsumerLab Tests
White Plains, NY, December 5, 2024 — Oats are a healthy source of fiber and naturally gluten-free. However, it’s not uncommon for oats to become contaminated with gluten during processing, which can cause problems for people with celiac disease and others who follow a gluten-free diet.
News Release
February 15, 2024
Unveiling the Truth About Green Tea’s EGCG Content
White Plains, NY, February 15, 2024 — ConsumerLab.com, a trusted name in health and wellness, sheds light on the EGCG (epigallocatechin gallate) content in green tea.
News Release
December 13, 2023
Best Vitamin C Supplements? ConsumerLab Selects Its Top Picks
White Plains, New York, December 13, 2023 — It’s officially cold season in the U.S., a time when many people may turn to vitamin C supplements to help “boost” their immune system and ward off colds.
News Release
March 30, 2023
ConsumerLab Tests Show Not All Quercetin and Rutin Supplements Contain What They Claim
White Plains, New York, March 30, 2023 — Quercetin is a plant compound found in its natural form, rutin, in foods such as capers, onions, and kale.
Recalls & Warnings
August 17, 2022
Seller of Moringa Tea and Other Herbal Products Warned for Drug Claims, Manufacturing Violations
On July 29, 2022, the FDA issued a warning letter to Deggeh Foods, Inc.
News Release
February 23, 2023
Best GABA Supplements Identified by ConsumerLab
White Plains, New York, February 23, 2023 — GABA (gamma-aminobutyric acid) supplements are claimed to have a "calming" effect, and are often promoted to reduce stress and insomnia and improve cognitive performance.
News Release
September 01, 2022
Big Differences in Strength of Olive Leaf Supplements in ConsumerLab Tests
White Plains, New York, September 1, 2022 — Unlike olive fruit and olive oil, olive leaf contains significant concentrations of oleuropein, a potentially beneficial compound. Olive leaf extract supplements are often promoted for lowering blood pressure and blood sugar.
Recalls & Warnings
June 17, 2024
Do Not Consume Diamond Shruumz Products, Warns CDC and FDA
On June 12, 2024, the Center for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), and America’s Poison Centers warned consumers not to buy or consume Diamond Shruumz chocolate bars, cones, and gummies.
News Release
July 20, 2022
Best Electrolyte Drinks for Hydration? Note: Some Contain 20 Times More Sodium Than Others
White Plains, New York, July 20, 2022 — It's important to stay well hydrated, and electrolyte products can help.
News Release
May 18, 2021
Caution With Potassium Supplements: You Could Get More Than Expected
White Plains, New York, May 18, 2021 — Recent tests by ConsumerLab.com of popular potassium supplements on the market revealed that consumers to get much more or much less potassium than expected from certain products.
News Release
April 17, 2021
ConsumerLab Identifies Best and Worst Avocado Oils Based on Testing
White Plains, New York, April 17, 2021 — Avocado oil has become increasing popular, but concern about the quality of products was raised in 2020 by a study from researchers at the University of California, Davis that found that as much as 82% of avocado oil samples bought locally or online in ...
News Release
June 06, 2020
Best Vitamin C Supplements Revealed by ConsumerLab
White Plains, New York, June 6, 2020 — Due to its role in maintaining immune system health, vitamin C has been promoted to help prevent and treat COVID-19, as well as other viral infections such as colds.
Recalls & Warnings
January 18, 2024
FDA Warns Consumers Not to Use Neptune’s Fix Products
On November 21, 2023, the FDA warned consumers not to purchase or use Neptune’s Fix products because they contain tianeptine. The FDA has received severe adverse event reports after use of these products, including seizure and loss of consciousness.
News Release
January 21, 2018
Best Apple Cider Vinegar? Beware of Poison! -- ConsumerLab.com Tests Reveal the Best and Worst Apple Cider Vinegars and Pills and Why Some Could Be Dangerous
White Plains, New York, January 21, 2018 — Apple cider vinegar is promoted as a safe and natural remedy for everything from lowering blood sugar and losing weight to improving digestion and treating infections.
News Release
May 17, 2017
Best Vitamin D? ConsumerLab.com Tests Popular Vitamin D Supplements and Reveals Its Top Picks
White Plains, New York, May 17, 2017 — Vitamin D is currently the most popular dietary supplement among people who regularly use supplements according to a recent survey by ConsumerLab.com.
Recalls & Warnings
February 24, 2025
Vitality Sexual Enhancement Supplement Recalled
On February 20, 2025, One Source Nutrition recalled all lots of Vitality capsules after FDA analysis found the supplements to contain undeclared prescription drugs sildenafil and tadalafil, which are not permitted in dietary supplements.
Recalls & Warnings
December 19, 2024
Gout and Arthritis Supplement Recalled Due to Undeclared Drugs
On December 12, 2024, Buy-herbal recalled all lots of Nhan Sam Tuyet Lien Truy Phong Hoan capsules within expiry after FDA testing found them to contain undeclared furosemide, dexamethasone and chlorpheniramine. These drugs are not permitted in dietary supplements.
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
Recalls & Warnings
April 30, 2021
FDA Warns Seller of Fish Oil, Apple Cider Vinegar, Collagen, and Elderberry Supplements
On April 13, 2021, the FDA issued a warning letter to Orphic Nutrition following a review of the company's websites, which found statements made about the products Apple Cider Vinegar Capsules, Ashwagandha Capsules, Garcinia Cambogia Capsules, Premium Collagen Peptides Capsules, Elderberry ...
Recalls & Warnings
November 16, 2023
Toxic Levels of Lead Found in Cinnamon Applesauce
WanaBana, Schnucks, and Weis apple cinnamon fruit puree pouches have been recalled after being linked, as of December 5, 2023, to 64 reports of illness, including four children with potential lead toxicity.
News Release
December 09, 2014
Round-up of Recent Product Tests by ConsumerLab.com -- Results for B Vitamin Supplements & Energy Drinks, Garlic, NAC (N-acetyl cysteine), Iron and Omega-7 Fatty Acids
White Plains, New York, December 9, 2014 — In recent months, ConsumerLab.com has published test results for a variety of popular supplements, including B vitamins supplements and energy drinks, NAC (N-acetyl cysteine), iron and garlic. In addition, ConsumerLab.
News Release
August 05, 2014
ConsumerLab.com Finds Popular Cocoa Powders Contaminated; High Concentrations of Cadmium Raise Concern
White Plains, New York, August 5, 2014 — Following up on its discovery in May of high levels of the toxic metal cadmium in several cocoa-based products, ConsumerLab.com recently tested additional, popular cocoa products sold in the U.S. to assess the extent of the problem.
News Release
March 05, 2013
CoQ10 & Ubiquinol Supplements Should Be Chosen Carefully, Cautions ConsumerLab.com -- Less Than 4% of Listed Ingredient Found in Widely-Sold Product
White Plains, New York — March 5, 2013 — A new report from ConsumerLab.com highlights the difficulty consumers have in selecting a supplement containing the anti-oxidant CoQ10 or its activated form, ubiquinol. ConsumerLab.
News Release
May 30, 2012
ConsumerLab.com Identifies Best and Worst Magnesium Supplements Based on Tests and Comparisons
White Plains, New York — MAY 30, 2012 — Magnesium supplements are among the most popular supplements in the U.S. But which magnesium supplements are best? ConsumerLab.
Recalls & Warnings
July 06, 2022
FDA Warns Kratom Companies for Arthritis, Pain Relief Claims & More
On January 30, 2022, the FDA issued warning letters to four sellers of kratom products because they were promoted to treat pain relief, opioid withdrawal, blood sugar control, anxiety, and to treat rheumatoid arthritis.
Recalls & Warnings
November 21, 2022
6 Supplement Companies Warned by FDA for Making Cholesterol Claims
On May 4, 2022, the FDA issued warning letters to six supplement companies following review that found statements made on company websites and Walmart purchase pages suggesting the products could lower cholesterol to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
March 17, 2025
Zaarah Herbal Powders Recalled Due to Lead, Arsenic Risk
On March 10, 2025, New York Wholesale Group recalled Zaarah Herbals Rasayan Churan, Zaarah Herbals Gurmar Powder, Zaarah Herbals Vasaka Powder and Zaarah Herbals Bhringraj Powder because they have the potential to be contaminated with elevated levels of lead and arsenic.
News Release
March 08, 2011
ConsumerLab.com reviews CoQ10 and ubiquinol supplements -- Tests show quality to be high, but large differences found in dosage, formulation, and cost
WHITE PLAINS, NEW YORK — MARCH 8, 2011 — A new report by ConsumerLab.com highlights the difficulty consumers may have in selecting a supplement containing the anti-oxidant CoQ10 or its activated form, ubiquinol. CoQ10 is among the most popular dietary supplements in the U.S.
News Release
January 25, 2011
ConsumerLab.com evaluates quality of potassium supplements and identifies those offering best value
WHITE PLAINS, NEW YORK — JANUARY 25, 2011 — A new report on the quality of potassium supplements was released by ConsumerLab.com today. In contrast to its last report on potassium supplements, ConsumerLab.
Recalls & Warnings
August 03, 2023
Arsenic Poisoning in U.S. from Ayurvedic Supplements
A 75-year-old woman in California experienced arsenic poisoning after taking Ayurvedic supplements prescribed to her by a holistic energy healer, according to a report published in July in Clinical Case Reports.
Recalls & Warnings
August 17, 2021
Weight Loss Supplements Found to Contain Sibutramine Recalled
Two companies (including one Ebay seller) recently recalled weight loss supplements that were found to contain sibutramine.
News Release
January 13, 2009
ConsumerLab.com reports largest test of CoQ10 and ubiquinol supplements -- Most supplements provide claimed ingredient, but large variation in dosage and forms may confuse consumers. ConsumerLab.com provides guidance.
WHITE PLAINS, NEW YORK — JANUARY 13, 2009 — A report by ConsumerLab.com on supplements containing the anti-oxidant CoQ10 shows the difficulty for consumers in determining an appropriate dosage.
News Release
April 16, 2008
FDA's investigation of generic antidepresssant called inadequate by ConsumerLab.com and The People's Pharmacy
WHITE PLAINS, NEW YORK AND DURHAM, NORTH CAROLINA — APRIL 16, 2008 — After waiting more than six months for results of the FDA's investigation of a generic version of Wellbutrin XL 300 (Budeprion XL 300), ConsumerLab.
News Release
March 17, 2008
As problems surface, Consumerlab.com and the people's pharmacy ask FDA to disclose differences between generic and original drugs
WHITE PLAINS, NEW YORK AND DURHAM, NORTH CAROLINA — MARCH 17, 2008 — An investigation by ConsumerLab.
News Release
January 08, 2008
ConsumerLab.com finds most potassium supplements meet quality standards, but one with only 18% of ingredient — Results for 12 potassium supplements in new review
WHITE PLAINS, NEW YORK — JANUARY 8, 2008 — A new report on the quality of potassium supplements was released by ConsumerLab.com today. Tests showed one product to contain only 18% of the claimed amount of this essential mineral.
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
Recalls & Warnings
March 02, 2022
Seller of Holistic Tea Warned by FDA
On February 17, 2022, the FDA issued a warning letter to Vital Health & Wellness following an inspection of the company’s website, which found statements made about the company’s product, Dr. Miller’s Holistic Premium Holy Tea, to be drug claims.
Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
News Release
October 20, 2006
ConsumerLab.com report on CoQ10 supplements finds quality high but large range in suggested dosage
WHITE PLAINS, NEW YORK — OCTOBER 20, 2006 — New test results from ConsumerLab.com for CoQ10 supplements show that all of the products selected contained amounts of ingredient consistent with their labels.
News Release
June 22, 2006
What's in a garlic supplement? Consumerlab finds few brands have it right and many mislead
WESTCHESTER COUNTY, NEW YORK — JUNE 22, 2006 A new study of garlic supplements by ConsumerLab.com found that only 6 of the 14 products it selected contained the amount of garlic expected from the labels and met quality standards.
Recalls & Warnings
January 12, 2021
Seller of Cholesterol, Weight Loss Products, and More Warned for Claims
On November 17, 2020, the FDA issued a warning letter to Bonagens following a review of the company's website, which found statements made about the company's products Anti-Aging Supports, Cholesterol & Diabetes Control, Gout Control, Hypertension Control, Liver Supports, Lungs ...
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
October 02, 2023
Dangerous Ingredients Found in Ten Arthritis and Pain Supplements
On September 29, 2023, the FDA warned consumers that the following arthritis and pain management products contain undeclared drugs such as diclofenac, methocarbamol (a muscle relaxant), and dexamethasone (a corticosteroid), and/or other potentially harmful ingredients.
Recalls & Warnings
April 23, 2025
FDA Warns Consumers Not to Use ADVANCED KING Joint Supplement
On February 10, 2025, the FDA warned consumers not to purchase or use the joint pain supplement ADVANCED KING (Naturista Store LLC), because FDA analysis confirmed the product contains dexamethasone, diclofenac, and methocarbamol.
Recalls & Warnings
January 28, 2025
Shatavari Powder Recalled Due to Risk of Lead Contamination
On January 27, 2025, New York Wholesale Group recalled Zaarah Herbals shatavari powder because of potential lead contamination.
Recalls & Warnings
February 03, 2025
Four Male Enhancement and Energy Supplements Found to Contain Drugs
On January 16, 2025, the FDA issued a Warning Letter to Mihon Corp. d/b/a VitalityVita and Boulla, LLC after FDA analysis found four of the company’s supplements, VitalityXtra, PeakMax, ZapMax, and ZoomMax, to contain undeclared sildenafil.
Recalls & Warnings
February 05, 2025
Muscle Tech Alpha Test Recalled
On December 18, 2024, Iovate Health Sciences USA Inc. recalled 10 lots (163,248 units) of Muscle Tech Alpha Test capsules because they contain cathine, a controlled substance that can cause serious adverse effects.
Recalls & Warnings
February 27, 2025
Male Enhancement Supplement Sold on Amazon and Walmart Recalled
On February 25, 2025, Natural Dior LLC recalled affected lots of a dietary supplement Vitafer-L Gold Liquid because it contains undeclared tadalafil, which is not permitted in dietary supplements.
Recalls & Warnings
May 01, 2024
FDA Warns Consumers Not to Use Ossos-Sans Supplement for Joints
On April 17, 2024, the FDA warned consumers not to purchase or use Ossos-Sans, a supplement promoted to treat osteoarthritis, osteoporosis, and other conditions, because it contains undeclared drugs, including diclofenac and methocarbamol.
Recalls & Warnings
June 12, 2024
Integrity Enhancement Supplements for Men Recalled Due to Undeclared Prescription Drugs
On February 1, 2024, Integrity Products issued a recall of one lot of Ram It & To The Moon products, which are promoted for sexual enhancement, after FDA analysis found the capsules to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
August 12, 2024
FDA Warns "Apricot Power" Apricot Seeds Contain Toxic Compound
On May 24, 2024, the FDA warned consumers not to consume three Apricot Power bitter apricot seed products because they were found to contain the toxic compound amygdalin.
Recalls & Warnings
August 20, 2024
Two Sexual Enhancement Supplements Sold on Amazon Recalled
On August 20, 2024, Veata LLC Endurance Pro Energy Boost capsules and Boulla LLC Boom Max capsules were recalled because they contain sildenafil, a prescription medication which is not permitted in dietary supplements.
Recalls & Warnings
October 08, 2024
AK Forte Joint Pain Supplement Recalled
On October 8, 2024, C&A Naturistics voluntarily recalled all lots of AK Forte, 400 mg tablets because FDA laboratory analysis found products to contain undeclared diclofenac, dexamethasone, and methocarbamol.
News Release
January 25, 2006
Sexual enhancement supplements analyzed by Consumerlab.com
WESTCHESTER COUNTY, NEW YORK — JANUARY 25, 2006 — ConsumerLab.com announced today that it found only six out of eleven supplements used for sexual enhancement to contain key ingredients listed on their labels and meet other quality criteria.
News Release
August 16, 2005
ConsumerLab.com tests potassium supplements — Report now online for sixteen products
WHITE PLAINS, NY — August 16, 2005 — ConsumerLab.com has released a new report on the quality of potassium supplements. Potassium is used to treat or prevent potassium deficiency caused by diuretic drugs ("water pills"), prolonged vomiting, diarrhea or laxative abuse.
News Release
October 29, 2002
Enormous variation found in strength of garlic supplements — most popular herb in U.S. — ConsumerLab.com releases results online today
WHITE PLAINS, NY — October 29, 2002 — ConsumerLab.com's Product Review of Garlic Supplements found strength to vary by as much as 1500% across products. Strength was based on each product's ability to generate allicin, a chemical associated with the efficacy of non-aged garlic.
News Release
May 05, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 5, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
News Release
March 06, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 6, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
February 02, 2021
FDA Warns Seller of Reishi Mushroom Supplements
On January 6, 2021, the FDA issued a warning letter to Entia Biosciences, Inc following a review of the company's website, which found statements made about the company's products Lion's Mane - All Natural Supplement, Antrodia Mushroom Powder, Maitake Mushroom Powder, Reishi ...
Recalls & Warnings
August 11, 2022
Problems With Supplements on Amazon
More than 50% of 30 top-listed immune support supplements purchased on Amazon.com in May of last year were found to list ingredients that could not be found in them with testing, according to a recent report (Crawford, JAMA Network Open, 2022).
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
June 23, 2003
FDA Warns Consumers Against Taking 6 Sexual Enhancement Supplements
On June 20, 2003, the Food and Drug Administration (FDA) warned consumers not to purchase or consume the following products: SIGRA, STAMINA Rx and STAMINA Rx for Women, Y-Y, Spontane ES and Uroprin, manufactured by NVE, Inc., in Newton, N.J. and distributed by Hi-Tech in Norcross, Ga.
Recalls & Warnings
September 12, 2018
FDA Urges Consumers to Avoid Kratom
On September 11, 2018, FDA Commissioner Scott Gottlieb, M.D., urged consumers not to use products containing the kratom, an herb that is often promoted for pain relief and for relieving symptoms of opioid withdrawal.
Recalls & Warnings
June 16, 2020
Seller of Silver, Arginine, and More Warned for Manufacturing Violations
On June 1, 2020, the FDA issued a warning letter to Morningstar Minerals LLC, which found the company's products, including Silver Boost, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
December 15, 2020
FDA Warns Seller of Evening Primrose Oil and Beta Glucan
On December 2, 2020, the FDA issued a warning letter to Smoky Mountain Naturals, LLC following a review of the company's website, which found statements made about the company's products Evening Primrose Oil and Beta Glucan to be drug claims.
Recalls & Warnings
November 24, 2020
Recalled Vitamin D Contains Potentially Dangerous Ingredient
On November 22, 2020, Fusion Health and Vitality LLC recalled all 2020 lots of CORE Essential Nutrients and Immune Boost Sublingual Vitamin D3 because they are adulterated. CORE Essential Nutrients contains the unapproved food additive hordenine HCl.
Recalls & Warnings
December 22, 2021
FDA Warns Seller of Liquid Magnesium, B-12, Berberine & More
On December 9, 2021, the FDA issued a warning letter to Wholly Liquid Nutritional Supplements LLC because it found statements on the company's website and social media about its products, including SpiroLaze, BioLaze, LiquiLaurin, VIT-B12, DeStress, and Omega Plus, to be ...
Recalls & Warnings
January 19, 2023
Male Sexual Enhancement Supplement Adam’s Secret Found to Contain Prescription Medication
On January 10, 2023, the FDA issued a warning letter to HIS Enterprise Inc dba Adam’s Secret USA, LLC after laboratory analysis found Adam’s Secret Extra Strength 3000 Platinum, Adam’s Secret Extra Strength Blue, Adam’s Secret Extra Strength Purple, Adam's Secret ...
Recalls & Warnings
April 27, 2022
Prescription Drugs Found in Arthritis, Muscle Pain, and Osteoporosis Supplements
On April 20th, 2022, the FDA warned consumers not to use the following ”Artri“ or ”Ortiga” products promoted to treat arthritis, muscle pain, osteoporosis and bone cancer because they have been found to contain undeclared drugs that can have serious adverse ...
Recalls & Warnings
June 14, 2023
Havasu Beet Root Powder Recalled Due to Allergen Risk
On June 12, 2023, Supplement Manufacturing Partner Inc. issued a recall of one lot of Havasu Nutrition’s Beet Root Powder + due to undeclared milk. The recall was initiated following a report of an allergic reaction from a consumer with a milk allergy.
Recalls & Warnings
May 04, 2023
PrimeZEN Enhancement Supplement for Men Found to Contain Prescription Drug
On April 27, 2023, the FDA issued a warning letter to Tager Online, Inc. DBA Volt Candy; Volt Candy Wholesale Club after FDA laboratory analysis determined PrimeZEN Black 6000, a product promoted to improve sexual function for men, contains tadalafil and sildenafil.
Recalls & Warnings
July 13, 2023
1st phorm Level-1 Protein Supplements Recalled Due to Staph Risk
On June 21, 2023, International Food Companies DBA International Food Products recalled two protein supplements due to potential contamination with Staphylococcus aureus.
Recalls & Warnings
July 26, 2023
Gadget Island, Inc. Male Enhancement Supplements Found to Contain Prescription Drugs
On July 21, 2023, the FDA issued a warning letter to Gadget Island, Inc.
Recalls & Warnings
August 25, 2023
FDA Warns Consumers Not to Use Big Guys Male Energy Supplement
On August 22, 2023, the FDA warned consumers not to buy or use BIG GUYS Male Energy Supplement after FDA laboratory analysis found it to contain undeclared sildenafil.
Recalls & Warnings
September 06, 2023
WEFUN Capsules Recalled Due to Undeclared Sildenafil
On August 25, 2023, WEFUN Inc. issued a recall of 300 boxes of WEFUN Capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
January 03, 2024
FDA Warns Amazon for Selling Men’s Supplements Containing Prescription Drugs
On December 20, 2023, the FDA issued a Warning Letter to Amazon.com, Inc.
Recalls & Warnings
November 30, 2023
Original The Rock Capsules Recalled Due to Undeclared Sildenafil
On October 18, 2023, Noah’s Wholesale, LLC issued a nationwide recall of one lot of the company’s Original The Rock capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
February 07, 2024
Sustain and Schwinnng Male Enhancement Supplements Recalled
On February 2, 2024, Today the World issued a recall of all lots of Sustain herbal dietary supplement capsules and one lot of Schwinnng capsules after FDA analysis found the supplements to contain undeclared prescription drugs tadalafil and nortadalafil.
Recalls & Warnings
March 21, 2024
Eleven Brands of Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On March 19, 2024, Pyramid Wholesale issued a recall of 11 brands of sexual enhancement supplements, including honey-based products, after they were found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
April 03, 2024
ForeverMen Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On February 12, 2024, ForeverMen issued a recall of all lots of ForeverMen capsules, which are promoted for sexual enhancement, after FDA analysis found the capsules to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
July 12, 2023
FDA Warns Company for Promoting Kratom Products for Pain, Mood, and More
On July 3, 2023, the FDA issued a warning letter to Sunshine Trading Company, Inc.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
August 30, 2012
FDA Warns of Medical Claims Made For Numerous Chinese Herbal Products
On August 15, 2012, the FDA warned Dragon Herbs that promotional statements made on the company's website constitute drug claims for twenty herbal products, including CardioPro 2000, Cordyceps, Duanwood Reishi, Gynostemma, Ginseng and Astragalus Combination, Dang Gui and Gelatin, Sweet Relief, ...
Recalls & Warnings
October 10, 2003
Government Stops Sale of Supplements Claiming to Treat Obesity and Impotence
On October 8, 2003, The Food and Drug Administration (FDA) announced that on September 22, 2003, U.S. District Court Judge Robert J. Vining, Jr.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
December 21, 2019
Supplement Company Continues to Make False Claims About Its Products, Says FTC
The FTC has filed a motion of contempt against two supplement companies that, according to the motion, have continued to promote their products with false claims despite being barred from doing so by a previous court order.
Recalls & Warnings
May 04, 2019
High Levels of Lead Found in Chinese Herbal Supplements
On May 2, 2019, Life Rising Corporation issued a recall Life Rising Holder-W Holder Warmer capsules, Life Rising NECK-ND Neck Clear capsules, and HoliCare Metabolism Cleansing (MET-CLS) tablets after FDA testing found high levels of lead in samples of the products.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Recalls & Warnings
February 25, 2022
Similac, Other Infant Formulas Recalled, Linked to Illness and One Death
Update: (3/7/22) This recall has been expanded to include one lot of Similac PM 60/40 following the death of an infant who tested positive for Cronobacter sakazaki and consumed Similac PM ...
Recalls & Warnings
July 06, 2021
CBD Products Were Promoted to Treat Cancer and Alzheimer's Without Proof, Says FTC
On July 6, 2021, the Federal Trade Commission (FTC) announced that it has approved a final administrative consent orders against Kushly Industries LLC and the company's owner, Cody Alt, for allegedly making unsupported health claims about its CBD products.
Recalls & Warnings
May 24, 2021
U.S. Marshals Seize $1.3 Million Worth of Kratom
U.S. Marshals have seized approximately $1.3 million worth of bulk kratom and kratom supplements manufactured by Atofil, LLC, a subsidiary of Premier Manufacturing Products, according to the FDA.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
November 17, 2022
FDA Warns Consumers Not to Use Male Enhancement Supplement
On November 10, 2022, the FDA warned consumers not to buy or use Sangter Natural Male Energy Supplement after FDA laboratory analysis confirmed the presence of undeclared sildenafil.
Recalls & Warnings
January 11, 2023
Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 9, 2022, the FDA issued a warning letter to Distributor RFR, LLC after laboratory analysis of the company’s SANGTER Natural Male Energy Supplement found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
February 13, 2023
FDA Finds Prescription Drug in “100% Natural” Weight Loss Supplement
On February 8, 2023, the FDA warned consumers not to buy or use Alfia 100% Natural Weight Loss Capsules after FDA laboratory analysis confirmed the presence of sibutramine.
Recalls & Warnings
July 01, 2022
Male Enhancement Supplements Sold on Amazon Recalled
On January 27, 2022, Loud Muscle Science LLC issued a voluntary recall of various lots of Launch Sequence supplements because they were found to contain the prescription drug tadalfil.
Recalls & Warnings
July 13, 2022
FDA Warns Sellers of Tainted Honey-Based Sexual Enhancement Products
On July 12, 2022, the FDA issued warning letters to four companies selling honey-based products promoted for sexual enhancement after tests conducted by the FDA found the products to contain the prescription drugs Tadalafil and Sildenafil.
Recalls & Warnings
July 15, 2022
Honey-Based Enhancement Supplement Recalled
On July 13, 2022, Shopaax.com issued a recall of all lots of its honey-based sexual enhancement product, Kingdom Honey Royal Honey VIP, after FDA analysis found the presence of undeclared sildenafil.
Recalls & Warnings
August 03, 2022
Sexual Enhancement Supplement Recalled Due to Sildenafil
On August 1, 2022, DISTRIBUTOR RFR, LLC recalled one lot of SANGTER Energy Supplement 3000 mg to the consumer level after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
July 26, 2022
Sexual Enhancement Supplement Sold on Amazon Recalled
On July 21, 2022, Ultra Supplements LLC issued a recall of one lot of Sustango capsules after Amazon laboratory analysis found the presence of the prescription drug Tadalafil. The company has received no reports of adverse effects related to this recall to date.
Recalls & Warnings
September 29, 2022
Sexual Enhancement Supplement Sold on Amazon and Walmart Recalled Due to Undeclared Drugs
On September 27, 2022, Proper Trade LLC/My Stellar Lifestyle recalled two lots of Wonder Pill after Amazon laboratory analysis found the product to contain undeclared tadalafil, a prescription medication.
Recalls & Warnings
September 06, 2022
Sand Ginger Powder Recalled in Canada Due to Aconite
On September 1, 2022, Ka Wing Hong issued a recall of Mr. Right brand Keampferia Galanga Powder (sand ginger powder), a common spice used in Asian cuisine, due to aconitine contamination.
Recalls & Warnings
September 07, 2016
Seller of Herbal Formulas for Cholesterol, Prostate & More Warned For Drug Claims
On July 29, 2016, the FDA issued a warning letter to Healing-Scents following a review of the company's website, which found statements made about its products, Heart Herbs, Cholesterol Regulation Herbs, Diabetes Regulation Herbs, Prostate Healer Herbs, High Blood Pressure Herbs, ...
Recalls & Warnings
April 21, 2020
Life Force Warned for Colloidal Silver, Nitric Oxide Claims
On March 27, 2020, the FDA issued a warning letter to Doctor's Signature Sales and Marketing International Corp.
Recalls & Warnings
January 28, 2020
Weight Supplement Contains Hidden Drug
On January 13, 2020, the FDA issued a warning letter to Wave Miami, LLC because the company's weight loss supplement, Lipro Dietary Capsule was found to contain the prescription medication tadalafil.
Recalls & Warnings
October 01, 2019
Lead and Arsenic Found in Multi Mineral & Vitamin Supplement
On September 30, 2019, Cellect Products Inc. and Oglethorpe Ltd. issued a recall of one lot of Cellect®, Essentials Factor® Multi Mineral & Vitamin Supplement Unflavored Powder Mix because it was found to contain unsafe levels of lead and arsenic.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
August 06, 2016
Some Supplements May Cause or Exacerbate Heart Failure (Includes vitamin E and many herbs)
Taking certain supplements may be dangerous for people with heart failure, according to a statement from the American Heart Association (AHA) which was published in the journal Circulation (August 2, 2016).
Recalls & Warnings
November 27, 2018
High Levels of Lead Found in Kratom Products
Some kratom products contain levels of lead and nickel that are not considered safe for human consumption, according to tests conducted by the FDA.
Recalls & Warnings
July 10, 2020
Sundial Herbal Recalls More Than Sixty Products Including Turmeric, Cinnamon & Tea
On July 9, 2020, Sundial Herbal Products issued a recall of more than 60 herbal products because they were misbranded and/ or promoted with drug claims. Their labels contain drug claims stating the products can diagnose, cure, mitigate, treat, or prevent disease.
Recalls & Warnings
June 30, 2020
FDA Warns Seller of Alpha Lipoic Acid, Berberine, and More
On June 18, 2020, the FDA issued a warning letter to Only Natural, Inc. dba Bio Nutrition, Inc.
Recalls & Warnings
April 05, 2022
Sexual Enhancement Capsules for Men Recalled
On April 1, 2022, F&S Medical Supply, dba Pink Toyz, recalled one lot of Pink Pussycat 3000 mg capsules to the consumer level because FDA analysis found them to contain sildenafil.
Recalls & Warnings
February 10, 2022
Three Sexual Enhancement Supplements Sold on Amazon Recalled
On February 8, 2022, specific lots of three male sexual enhancement supplements, Red Mammoth capsules, MAC DADDY RED capsules, MAC DADDY PURPLE capsules, and The Red Pill capsules, were recalled because Amazon laboratory analyses found them to contain undeclared ...
Recalls & Warnings
November 05, 2021
Herb Recalled Due to Risk of Lead, Cadmium Contamination
On November 2, 2021, Murray Int'l Trading issued a recall of Herbal Doctor brand Angelicae Sinensis due to potential risk it may contain elevated levels of lead and cadmium.
Recalls & Warnings
July 27, 2021
"Miss Slim" Capsules Recalled
On July 20, 2021, HIS issued a recall of all lots and presentations of Miss Slim capsules because FDA analysis found them to contain sibutramine.
Recalls & Warnings
July 20, 2021
Alpha Male Plus Recalled
On July 16, 2021, Alpha Male Plus issued a recall of Alpha Male Plus Male Enhancer fruit chews because FDA analysis found them to be contaminated with tadalafil.
Recalls & Warnings
April 26, 2021
Family Indicted for Selling Dangerous "Bleach" Miracle Mineral Solution As "COVID" Cure
Four Florida men have been indicted by a Miami federal grand jury on charges of fraudulently marketing and selling the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions, such as cancer, Alzheimer's, diabetes, autism, malaria, ...
Recalls & Warnings
June 22, 2011
Recall of Calcium & Magnesium Softgels Containing Excessive and Potentially Toxic Amounts of Vitamin D
On or about June 7, 2011, NOW Foods announced the following recall to retailers. ConsumerLab.com was subsequently made aware of the recall by a ConsumerLab.com member.
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
Recalls & Warnings
May 29, 2003
Recall and Warning for Another Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On May 23, Best Life International, in cooperation with the U.S. Food and Drug Administration, warned consumers not to purchase or consume the product known as Viga.
Recalls & Warnings
April 09, 2003
Recall of Dangerous Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On April 4, 2003, the U.S. Food and Drug Administration's MedWatch program announced that Ultra Health Laboratories, Inc. and Bionate International, Inc. are warning consumers not to purchase or consume a product known as Vinarol tablets.
Recalls & Warnings
January 24, 2013
Food for Health Warned for Manufacturing Violations and Drug Claims on Supplements
On October 5, 2012, the FDA issued a warning letter to Food for Health International, LLC following a facility inspection which found the company's products, including Activz brand Vitamin D, Potassium Iodine, Organic Vitamin C, Whole 9 (a fruit and vegetable meal replacement shake) Control and VMA ...
Recalls & Warnings
March 09, 2021
CBD Seller Warned for Drug and COVID-19 Claims
On March 1, 2021, the FDA issued a warning letter to Cannafyl following a review of the company's website, which found statements made about the company's products Balance CBD Drops, Relief CBD Drops, Relax CBD Drops and Relief CBD Salve to be drug claims.
Recalls & Warnings
April 21, 2012
FDA Finds Prescription Drug Analog in 3 Enhancement Supplements
On April 20, 2012, the U.S. FDA advised consumers not to purchase or use the following supplements from Enhance Nutraceutical: "Instant Hard Rod," "RigiRx Plus," and "Zen Maxx." FDA laboratory analysis confirmed that each contains aminotadalafil.
Recalls & Warnings
April 03, 2012
"France T253" Enhancement Supplement Contains Hidden Drug
April 3, 2012: The U.S. Food and Drug Administration (FDA) is advising consumers not to purchase or use “France T253,” a product for sexual enhancement. This product is promoted and sold on various Web sites, such as ebay.com.
Recalls & Warnings
May 17, 2012
FDA Warns of Drug Ingredients in Three Sexual Enhancement Supplements
On May 16, 2012, the U.S. FDA advised consumers not to purchase or use the following three sexual enhancement products: Boost - Ultra Sexual Enhancement Formula (sold on www.boostultra.biz), Firminite (sold on www.firminite.com), and VMaxx Rx (sold on www.vmaxxrx.com).
Recalls & Warnings
January 11, 2012
Herbal Extract Company Warned by FDA of Making Drug Claims
On January 3, 2012, the U.S. FDA sent a Warning Letter to Herbal Extract Plus, LLC regarding legal violations for claiming its herbal products can cure or prevent diseases.
Recalls & Warnings
September 13, 2010
Supplement for "Increasing Desire" Recalled for Containing Drug
The U.S. FDA posted a notice regarding the recall of Masxtreme Capsules by the distributor, Natural Wellness, Inc. FDA analysis has determined the product contains undeclared amounts of Aminotadalafil, an analog of tadalafil.
Recalls & Warnings
April 08, 2010
Capsule for Men Spiked with Erectile Drug
On April 7, 2010, the U.S.
Recalls & Warnings
July 20, 2010
Recall of ED Supplement Containing Drug
On July 20, 2010, the U.S. FDA posted a news announcement from Good Health, Inc. regarding the recall of Vialipro, a sexual enhancement supplement sold nationally.
Recalls & Warnings
July 23, 2009
Six Male Enhancement Supplements Found Adulterated
On July 15, 2009, the U.S. FDA announced that Obteron 1 Inc. dba Nature & Health Co. is recalling the following supplements: LibieXtreme, Y-4ever, Libimax X Liquid, Powermania Capsule, Powermania Liquid, and Herbal Disiac.
Recalls & Warnings
February 07, 2012
Drug-Laced Supplements and Drink Mixes Recalled
According to the U.S. FDA, on February 3, 2012 Healthy People Co. announced a voluntary nationwide recall of several dietary supplements sold as capusules, drinks, and shake mixes. The supplements were found to contain drug substances and pose risks.
Recalls & Warnings
February 24, 2011
Recall of Counterfeit Extenze Tablets Containing Drugs
On February 23, 2011, the FDA announced that Biotab Nutraceuticals, Inc. was notified that two lots of counterfeit product purporting to be Extenze contain undeclared drug ingredients. Specifically, lot 0709241 contains tadalafil and sildenafil, and lot 0509075 contains tadalafil and sibutramine.
Recalls & Warnings
March 08, 2012
Rx Drugs Found in Weight Loss and Enhancement Supplements
On February 6, 2012, FDA issued a letter to Globe All Wellness, LLC warning that two of their products contain undeclared prescription drugs.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
April 16, 2014
Seller of Aloe, Thyroid and Other Supplements Warned for Manufacturing Violations and Drug Claims
On March 21, 2014, the FDA issued a warning letter to Aloe Man International Corp.
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.
Recalls & Warnings
October 04, 2017
Police Officer Dies From Kratom Overdose, Herb Currently Legal in Most States
A police officer who worked in narcotics in Tupper Lake, New York, died this summer of an overdose of the herb kratom, according to a report from ABC News.
Recalls & Warnings
April 01, 2004
Aloe Producer Recalls Product Due to Toxic Levels of Vitamin D
On March 26.2004, the Food and Drug Administration (FDA) announced that Aloe Commodities International, Inc., Carrollton, Texas, is recalling 1600 bottles of Solutions IE Ageless Formula II, Lot numbers P2207 and P2221 because they contain a significantly higher-than-labeled level of vitamin D3.
Recalls & Warnings
November 24, 2018
Dangerous Drug In Supplements for Pain and Anxiety
On November 20, 2018, the FDA announced it has issued warning letters to two companies for the illegal marketing of products labeled as dietary supplements that contain the opioid-like drug tianeptine.
Recalls & Warnings
September 09, 2018
Higenamine -- A Potentially Dangerous Stimulant -- Found in Some Supplements
A recent study found concerningly high doses of the stimulant higenamine in some weight loss, energy and sports supplements sold in the U.S. Higenamine is a naturally-occurring stimulant which is permitted to be sold as a dietary supplement ingredient in the U.S.
Recalls & Warnings
February 17, 2021
Adam’s Secret Male Enhancement Products Recalled Due to Undeclared Drugs
On February 15, 2021, adamssecret.co issued a recall of all lots of Adam's Secret Extra Strength 1500 and Adam's Secret Extra Strength 3000 male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
December 15, 2020
FDA Warns JC Ayur Life LLC for Drug Claims
On October 29, 2020, the FDA issued a warning letter to JC Ayur Life LLC following a review of the company's website, which found statements made about the company's product Heritage of Ayurveda Dia-Tonic Incudil Herbal Dietary Supplement to be drug claims.
Recalls & Warnings
April 17, 2021
NS NY Distributor Inc Male Enhancement Products Recalled Due to Undeclared Drugs
On April 8, 2021, NS NY Distributor Inc issued a recall of all lots of Premium Orgazen 7000 and Ginseng Power 5000 male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
April 10, 2021
Three More Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 30 and April 5, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
April 02, 2021
Three Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 24 and 26, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and tadalafil.
Recalls & Warnings
September 03, 2020
Red-E Male Enhancement Capsule Recalled
On September 1, 2020, The Protein Shoppe, LLC issued a recall of Red-E male enhancement capsules because FDA analysis found it to contain sildenafil.
Recalls & Warnings
March 25, 2008
FDA Warns of Sexual Supplements with Drug-like Ingredients
On March 25, 2008, the U.S. Food and Drug Administration advised consumers not to purchase or use "Blue Steel" or "Hero" products marketed as dietary supplements throughout the United States because they are considered unapproved drugs and have not been proven to be safe or effective.
Recalls & Warnings
May 10, 2007
FDA Warns of Two Supplements Containing Pharmaceutical-like Compounds
On May 10, 2007, the FDA advised consumers not to purchase or use "True Man" or "Energy Max" products promoted and sold as dietary supplements throughout the United States.
Recalls & Warnings
May 06, 2015
Joint Pain Supplements Found to Contain Diuretics, Antihistamines and Other Undeclared Drugs
On May 4, 2015, the FDA warned consumers not to buy or use the following supplements promoted for joint or back pain because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
August 01, 2017
"Man of Steel" Sexual Enhancement Supplements Recalled
On July 27, 2017, Man of Steel recalled its Man of Steel 1 and Man of Steel 2 sexual enhancement supplements because they were found to contain undeclared sildenafil.
Recalls & Warnings
April 29, 2014
Seller of B Vitamins, CoQ10, Amino Acids and Other Supplements Warned for Manufacturing Violations and Drug Claims
On April 1, 2014, the FDA issued a warning letter to Bio-Recovery, Inc.
Recalls & Warnings
March 02, 2012
Recall of Enhancement Supplement for Men -- Contains Drug
On Feb, 24, 2012, Regeneca, Inc. announced a voluntary nationwide recall of all lots of RegenErect. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a blue capsule sold individually in foil packets with a UPC code of 816860010055.
Recalls & Warnings
January 01, 2011
Recall of Two Supplements Spiked with Erectile Dysfunction Drug
On December 22, 2010 the U.S.
Recalls & Warnings
February 16, 2012
Recall of Enhancement Supplement for Women -- Contains Drug
On Feb, 10, 2012, Regeneca, Inc. announced a voluntary nationwide recall of RegenArouse Natural Female Intimacy Enhancement. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a pink capsule sold individually in foil packets.
Recalls & Warnings
July 19, 2011
Recall of Enhancement Supplement for Men - Contains Drug Compounds
On July 13, 2011, Global Wellness, LLC announced an expanded voluntary nationwide recall of Via Extreme Ultimate Sexual Enhancer Dietary Supplement for Men. The product was distributed throughout the U.S. Puerto Rico, Barbados, and Canada to internet and retail consumers.
Recalls & Warnings
April 07, 2010
Nationwide Recall of Masxtreme Capsules Containing Drugs with Cardiovascular Side Effects
On March 30, 2010, the U.S. Food and Drug Administration (FDA) posted a notice from Natural Wellness warning consumers not to purchase or consume the product known as MasXtreme, Lot# 911035.
Recalls & Warnings
December 14, 2009
Nationwide Recall of Sexual Enhancement Supplements Containing Drug-like Compound
On December 14, 2009, the Federal Drug Administration (FDA) posted a notice on its website regarding the recall of many sexual enhancement supplements sold by Atlas Operations, Inc.
Recalls & Warnings
September 12, 2010
Mr. Magic Male Enhancer Recalled
The U.S. FDA posted a recall announcement daated August 18, 2010 from Glow Industries regarding "Mr. Magic Male Enhancer from Don Wands.
Recalls & Warnings
August 13, 2010
Recall of Male Enhancement Supplement Sold in National Stores
On August 9, 2010 the U.S. FDA announced that the company Prolatis' of Salt Lake City, Utah is conducting a voluntary recall of the company's product sold as Prolatis'.
Recalls & Warnings
March 20, 2007
Recall of Adulterated Sexual Enhancement Supplement
As posted on the FDA website, on March 15, 2007, the company Barodon SF of Los Angeles, CA announced that it is conducting a voluntary nationwide recall of the company's supplement product sold under the name V.MAX.
Recalls & Warnings
November 26, 2007
Encore Tabs Recalled -- Contain Prescription Drug
On November 21, 2007, Bodee LLC announced today that it is conducting a voluntary nationwide recall of all the company's supplement product sold under the name Encore Tabs.
Recalls & Warnings
March 04, 2008
Two Supplements Recalled for Containing Viagra-like Compounds
On March 4, 2008, the FDA posted a release from Palo Alto Labs (dated February 28) announcing a voluntary nationwide recall of the company's supplement products sold under the name Aspire36 and Aspire Lite.
Recalls & Warnings
May 29, 2008
Recall of Virility Supplement
On May 29, 2008, the FDA posted a recall by International Pharmaceuticals, Ltd. of all of the company's supplement sold under the name of Viril-Ity-Power (VIP) Tabs, 560mg/serving.
Recalls & Warnings
July 30, 2008
Recall of Viapro Capsules Due to Potentially Harmful Ingredient
The U.S. FDA has posted a release noting that EG Labs, LLC announced a nationwide voluntary recall on July 23, 2008 of all lots of its supplement product sold under the brand name, Viapro, in 375mg capsules.
Recalls & Warnings
November 03, 2004
FDA Warns of Sexual Enhancement Supplements Containing Prescription Drug
On November 2, 2004, the Food and Drug Administration (FDA)warned consumers not to purchase or to consume Actra-Rx or Yilishen, two products promoted and offered for sale on Web sites as "dietary supplements" for treating erectile dysfunction and enhancing sexual performance for men.
Recalls & Warnings
October 24, 2012
Manufacturer of Green Tea, Vitamin E, Omega-3 and Cranberry Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On October 16, 2012, the FDA issued a warning letter to dietary supplement manufacturer Advanced Nutritional Technology Inc.
Recalls & Warnings
September 18, 2012
Maker of Noni, Nopal, Blood Sugar and Cholesterol Supplements Warned For Drug Claims, Misbranding, and Manufacturing Violations
On August 27, 2012, the FDA issued a warning letter to Naturavit, Inc. for making statements about dietary supplements Noni Imperial Hawaiian, Garlic and Parsley, Cholestol, Nopal and Diatrin that constitute drug claims.
Recalls & Warnings
September 13, 2010
ExtenZe Enhancement Supplements Seized in Canada
On August 19, 2010, Health Canada (Canada's health ministry) seized the sexual enhancement supplements "Male Enhancement ExtenZe" and "Women ExtenZe" which were imported from the U.S. Although legal and widely sold in the U.S.
Recalls & Warnings
January 17, 2018
Joint and Pain Relief Product Contains Undeclared Drug
On January 17, 2018 Health Canada warned consumers that Linsen Double Caulis Plus, a supplement for joint pain, was found to contain the undeclared drug dexamethasone.
Recalls & Warnings
January 18, 2003
Non-Prescription Anti-Hypertension Pills Recalled
On January 17, the U.S. FDA's MedWatch Program reported that Herbsland Inc.
Recalls & Warnings
March 28, 2011
Dangerously High Levels of Vitamins A and D in Product Prompt FDA Warning
On March 28, the U.S. FDA warned consumers to stop using Soladek, a vitamin-solution product marketed by Indo Pharma, S.A., of the Dominican Republic, because the product may contain dangerously high levels of vitamins A and D.
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
Recalls & Warnings
July 13, 2006
FDA Warns About Sexual Enhancement Supplements with Dangerous Ingredients
On July 12, 2006, the Federal Drug Administration (FDA) warned consumers not to purchase or consume Zimaxx, Libidus, Neophase, Nasutra, Vigor-25, Actra-Rx, or 4EVERON.
Recalls & Warnings
May 28, 2008
FDA Requests Recall of Xiadafil VIP Supplements
On May 27, 2008, the U.S. The U.S. Food and Drug Administration announced that it had requested that SEI Pharmaceuticals, of Miami, Fla.
Recalls & Warnings
December 30, 2007
FDA Warns Consumers Not to Use Several "Shangai" Sexual Enhancement Supplements
On December 28, 2007, the U.S. Food and Drug Administration (FDA) advised consumers not to buy or use Super Shangai, Strong Testis, Shangai Ultra, Shangai Ultra X, Lady Shangai, and Shangai Regular, also marketed as Shangai Chaojimengnan, products.
Recalls & Warnings
July 29, 2008
Recall of Sexual Enhancement Supplements Containing Drug
On July 28, 2008 the U.S. FDA announced that Jack Distribution, LLC and G & N works, Inc. are conducting a voluntary nationwide recall of all lot numbers of the company's supplement products sold under the brand names Rize 2 The Occasion and Rose 4 Her.
Recalls & Warnings
April 19, 2007
Recall of Another Sex Enhancement Supplement
On April 17, 2007 the Food and Drug Administration (FDA) website posted an announcement that Jen-On Herbal Science International, Inc. is conducting a voluntary nationwide recall of the company's supplement product sold under the name H S Joy of Love. Jen-On Herbal Science International, Inc.
Recalls & Warnings
December 08, 2009
Warning on Acai Berry Supplements Spiked with Drug
On December 8, 2009, Health Canada (the Canadian health agency) advised consumers not to use certain Acai Berry products after a large number of shipments of adulterated products were stopped at the border.
Recalls & Warnings
June 15, 2009
Sexual Enhancement Supplement Recalled -- Second Time Found with Drug-like Compound
On June 15, 2009, the U.S. FDA announced that, per its order, Hi-Tech Pharmaceuticals, Inc. is conducting a nationwide voluntary recall of the company's product sold under the name Stamina-Rx.
Recalls & Warnings
November 21, 2012
Maker of Blood Pressure, Probiotic, Resveratrol and Hormonal Supplements Warned For Manufacturing Violations and Drug Claims
On November 2, 2012, the FDA issued a warning letter to Atrium, Inc. following a facility inspection which found a number of the company's dietary supplements to be adulterated or promoted as drugs.
Recalls & Warnings
August 30, 2006
FDA Warns that Dietary Supplements Containing Ephedrine Alkaloids are Illegal and Pose Risk to Users
On August 21, 2006 the US Food and Drug Administration (FDA) issued a statement on the Tenth Circuit’s ruling to uphold the FDA decision banning dietary supplements containing ephedrine alkaloids.
Recalls & Warnings
April 07, 2011
Prostate Drug Found in Prostate Supplement -- Recall Underway
On March 24, 2011 the U.S. FDA posted a voluntary recall notice for U-Prosta from USA Far Ocean Group, Inc.
Recalls & Warnings
December 21, 2012
Dangerous Supplement Being Sold Under New Name
On December 21, 2012, the FDA warned consumers that Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain that has been linked to serious and sometimes fatal side effects, is now being sold under the name WOW.
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
November 28, 2015
Herbal Supplements Linked to Heavy Metal Poisoning
On November 17, 2015, Health Canada (the Canadian equivalent of the FDA) warned that a consumer in Canada who purchased and used herbal supplements from a U.S.-based website was treated for heavy metal poisoning.
Recalls & Warnings
September 18, 2015
High Levels of Mercury and Lead Found in Ayurvedic Herbal Supplements
On September 17, 2015, the FDA announced that Shree Baidyanath Ayurved Bhawan Ayurvedic dietary supplements were found to contain high levels of lead and/or mercury, which can cause serious health problems.
Recalls & Warnings
October 30, 2008
Possible Risk Rather than Benefit Found in Trial of Vitamin E and Selenium for Prostate Cancer
On October 27, 2008 the National Cancer Institute (NCI) announced that men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) are being told to stop taking their supplements.
Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
January 29, 2019
Prescription Drugs Found in Four Weight Loss Supplements
On January 28, 2019, the FDA the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine and/or phenolphthalein:
Recalls & Warnings
March 30, 2019
FDA Warns Seller of Reishi, Joint Supplements, Ginseng & More for Pesticides, Other Violations
On March 12, 2019, the FDA issued a warning letter to Yanqing "Michael" Li and Chang Su, owners of the website http://www.woohoonatural.
Recalls & Warnings
September 21, 2019
Liquid Vitamin C for Men Recalled
On September 16, 2019, Fitoterapia USA Inc. issued a recall of Macho Artificial Passion Fruit Flavored Vitamin C Liquid Supplement, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
August 12, 2019
Miracle Mineral Solution Is Dangerous, Warns FDA
On August 12, 2019, the FDA warned consumers not to buy or use Miracle Mineral Solution or other "Miracle" or "Master" solution products containing sodium chlorite because they can dangerous and even life-threatening reactions.
Recalls & Warnings
July 23, 2019
FDA Warns Seller of CBD Lotions, Patches, Tinctures and Pet Products
On July 22, 2019, the FDA issued a warning letter to Curaleaf, Inc.
Recalls & Warnings
May 15, 2019
Titanium 4000 Capsules for Men Recalled
On May 7, 2019, D.B.P. Distribution issued a recall of Titanium 4000, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
May 07, 2019
"The Beast" Supplement for Men Recalled
On May 7, 2019, STIFF BOY LLC issued a recall of The Beast, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
December 21, 2019
Men's Enhancement Supplement Recalled
On December 17, 2019, Motto International Corp all lots of Bull Platinum 30000, Stallion Platinum 30000, Rhino 7 Platinum 30000, and Panther Platinum 30000, to the consumer leve because FDA analysis has found they contain tadalafil.
Recalls & Warnings
March 17, 2020
"Active Male" Supplement Recalled
On March 16, 2020, Natural Remedy Store recalled all lots of Active Male, 500mg, to the consumer level because FDA analysis has found the product contains tadalafil.
Recalls & Warnings
November 16, 2019
Silver Bullet Supplement for Men Recalled
On November 13, 2019, Nature's Rx issued a recall of one lot of Silver Bullet male enhancement capsules because FDA analysis found it to contain sildenafil.
Recalls & Warnings
November 12, 2019
Libido Supplement for Men and Women Recalled
Update: (2/25/20) This recall has been expanded to include lots of an additional Med Man product, Bow and Arrow libido enhancer for men, highlighted below.
Recalls & Warnings
October 26, 2019
Green Lumber Enhancement Supplements Recalled
On October 22, 2019, GL Holdings issued a recall of Green Lumber, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
June 25, 2019
Kratom Is Dangerous and Not Proven to Treat Addiction, FDA Warns Marketers
On June 25, 2019, the FDA issued warning letter to two marketers and distributors of kratom products, listed below, for illegally selling unapproved, misbranded kratom-containing drug products with unproven claims about their ability to treat or cure opioid addiction and withdrawal symptoms.
Recalls & Warnings
March 19, 2007
Canada Warns of Weight Loss Supplement with Rx Drug
On March 14, 2007, Health Canada advised consumers not to use MIAOZI Slimming Capsules because they have been found to contain sibutramine, a prescription medication that should only be taken under medical supervision.
Recalls & Warnings
July 25, 2015
Weight Supplement Found to Contain Three Drugs
On July 23, 2015 Life & More, L.L.C. issued a voluntary recall of 783 bottles from one lot of Akttive High Performance Fat Burner Gold weight loss capsules because they were found to contain the undeclared drugs sibutramine, desmethylsibutramine, and phenolphthalein.
Recalls & Warnings
October 24, 2015
Eight Enhancement Supplements Found to Contain Undeclared Drugs
On October 23, 2015, the FDA warned consumers not to buy or use the following three sexual enhancement supplements because they were found to contain an undeclared sildenafil or similar drugs (some were identified during an examination of international mail shipments):
Recalls & Warnings
April 17, 2015
Herbal Laxative and "Detox" Kit Containing High Levels of Lead and/or Arsenic Recalled in Canada
On April 15, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that St. Francis Herb Farm Inc.
Recalls & Warnings
August 08, 2016
Turmeric Spice Recall Expanded to Include More Brands
On August 5, 2016, Gel Spice, Inc. expanded its recall of one lot of Fresh Finds Ground Turmeric Powder to include other turmeric spices sold under different brand names due to elevated levels of lead.
Recalls & Warnings
November 14, 2014
FDA Warns Consumers Not to Buy or Use Muscle Enhancement Supplement
On November 13, 2014, the FDA advised consumers not to buy or use the muscle enhancement supplement Mayhem (Chaotic-Labz) because it was found to contain the undeclared drugs cyproheptadine and dexamethasone. These drugs can cause serious side effects and can interact with other medications.
Recalls & Warnings
July 24, 2014
Seller of Noni Juice Products Warned for Manufacturing Violations, Drug Claims
On July 14, 2014, the FDA issued a warning to Noni Connection dba Puna Noni, following a facility inspection which found the company's Puna Noni 100% Pure Hawaiian Noni Fruit Capsules to be adulterated because they were prepared, packed, or held under conditions that violate Current Good ...
Recalls & Warnings
July 29, 2016
Ground Turmeric Spice Recalled Due to Lead Contamination
On July 28, 2016, Gel Spice, Inc. issued a voluntary recall one lot of Fresh Finds Ground Turmeric Powder because it was found to contain elevated levels of lead.
Recalls & Warnings
February 20, 2013
Recall: Arthritis and Muscle Pain Supplement Contains Prescription Drugs
On February 15, 2013, Reumofan Plus USA, LLC and Reumofan USA, LLC announced a recall of Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain, because it was found to contain the active pharmaceutical ingredients methocarbamol, dexamethasone, and diclofenac.
Recalls & Warnings
February 13, 2013
Manufacturer of Heart, Multivitamin, Cranberry, and Longevity Supplements Warned For Adulteration and Drug Claims
On January 29, 2013, the FDA issued a warning letter to M.D.R. Fitness Corp. following a facility inspection which found violations of good manufacturing practices which cause the company's products to be adulterated.
Recalls & Warnings
November 26, 2012
Court Injunction Halts Sale of Cholesterol, CoQ10, Progesterone, Prostate Supplements and More
On November 20, 2012, Pharmacist's Ultimate Health agreed to cease sales and distribution of its dietary supplements by entering into a Consent Decree of Permanent Injunction in the U.S. District Court of Minnesota.
Recalls & Warnings
September 30, 2015
Weight and Energy Supplement Contains Synthetic "Amphetamine"
On September 21, 2015, the FDA issued a warning letter to TruVision Health LLC. because the company's supplement tru Weight & Energy lists a synthetic, amphetamine-like compound, AMP (also called 1,3-dimethylbutylamine or DMBA) on product labels.
Recalls & Warnings
November 11, 2015
Consuming a Single Energy Drink May Increase Cardiovascular Risk
A recently published study found that consumption of a single caffeinated energy drink significantly increased blood pressure and noradrenaline levels in healthy young adults. The researchers noted these changes could potentially increase the risk of cardiovascular events.
Recalls & Warnings
December 14, 2010
FDA Warns Consumers to Avoid Man Up Now Capsules
On December 14, 2010, the U.S. FDA warned consumers not to use Man Up Now capsules, marketed as a dietary supplement for sexual enhancement, because they contain a variation of an active drug ingredient found in Viagra that can dangerously lower blood pressure.
Recalls & Warnings
October 15, 2007
US Marshalls Seize Charantea Supplements Promoted for Diabetes, Anemia and Hypertension
At the request of the U.S. Food and Drug Administration (FDA), on October 9, 2007, U.S. Marshals seized approximately $71,000 of goods from FulLife Natural Options, Inc., of Boca Raton, Fla., which marketed and distributed Charantea Ampalaya Capsules and Charantea Ampalaya Tea.
Recalls & Warnings
October 08, 2010
FDA Warns of Stimulant in Slimming Capsules
On October 8, 2010, the U.S. FDA advised consumers who have Slimming Beauty Bitter Orange Slimming Capsules not to use the product. FDA warns that Slimming Beauty Bitter Orange Slimming Capsules contain the active pharmaceutical ingredient sibutramine, a prescription-only drug which is a stimulant.
Recalls & Warnings
August 28, 2008
FDA Finds Fault with Generic Toprol XL -- Problems Reported Earlier by ConsumerLab.com
On August 12, 2008, the U.S. FDA sent a letter to Sandoz Inc. warning of violations in its manufacture of Metoprolol Succinate ER tablets and other drug products. Sandoz's Metoprolol Succinate ER tablets are generic versions of Toprol XL.
Recalls & Warnings
October 31, 2017
Black Licorice Can Cause Abnormal Heart Rhythms, FDA Warns
On October 30, 2017, the FDA warned consumers that eating 2 ounces of black licorice a day for at least two weeks could cause an irregular heart rhythm or arrhythmia in adults age 40 or older.
Recalls & Warnings
August 05, 2017
Children's Ayurvedic Medicine Formula Contains High Levels of Lead
On August 4, 2017, the FDA warned parents and caregivers not to use Balguti Kesaria Ayurvedic Medicine due to the risk of lead poisoning.
Recalls & Warnings
January 30, 2013
Seller of Omega-3 and Emu Oil Supplements Warned For Drug Claims, Misbranding
On November 19, 2012, the FDA issued a warning letter to Emu Products & Management, Inc. after a review of the company's website found statements that promoted the dietary supplements Multi-Omega Gel Caps, Recovery Gel Caps, ARP Gel Caps and the topical skin products Extreme Cryo Gel, F.A.C.E.
Recalls & Warnings
February 07, 2013
Growth Factor Supplement For "Size, Strength and Stamina" Recalled Due To Potential Contamination
On January 2, 2013, EonNutra, LLC issued a voluntary recall of specific batches of Soto Supplements Growth Factor Complex 200 GFC 200 (2 fl oz.) liquid dietary supplement due to potential bacterial contamination.
Recalls & Warnings
December 12, 2015
Sexual Enhancement Supplement Recalled
On December 11, 2015, Reesna Inc. issued a recall of its sexual enhancement supplements Fuel Up Plus and Fuel Up High Octane which were distributed in August 2015 and were found to contain undeclared hydroxythiohomosildenafil.
Recalls & Warnings
March 09, 2016
Four Sexual Enhancement Supplements Recalled
On March 7, 2016, Health Canada (the Canadian equivalent of the FDA) announced that Vivo Brand Management is recalling four sexual enhancement products sold in Canada which may contain the undeclared drug sildenafil. Some of these products also appear to be available for purchase in the U.S.
Recalls & Warnings
August 31, 2016
Recall of Dangerous Chinese Herbal Supplement Expanded
On August 30, 2016, Ton Shen Health expanded its initial recall of DHZC-2 tablets to include all lots purchased before August 24, 2016, due to potential contamination with elevated levels of lead.
Recalls & Warnings
August 16, 2016
Chinese Herbal Supplement Recalled Due to Lead Contamination
On August 11, 2016, Ton Shen Health issued a recall its DHZC-2 tablets due to potential contamination with elevated levels of lead.
Recalls & Warnings
August 05, 2016
More Turmeric Spice Recalled
On August 5, 2016, JM Exotic Foods, Inc. issued a recall of ground turmeric because it was found to contain elevated levels of lead.
Recalls & Warnings
July 29, 2017
Increase in Calls to Poison Control Centers About Supplements
The number of calls to poison control centers in the U.S. about dietary supplement exposures increased by almost 50% between 2005 and 2012, according to a study published this week in the Journal of Medical Toxicology.
Recalls & Warnings
July 10, 2017
Bodybuilding Supplements Containing Steroid-Like Substances Recalled
On June 29, 2017, Hardcore Formulations recalled all lots and expiration dates of their products Ultra-Sten and D-Zine, bodybuilding supplements found to contain methylstenbolone and dymethazine, which are derivatives of anabolic steroids.
Recalls & Warnings
April 04, 2017
Seller of B Vitamins, Vitamin C, Potassium & More Warned for Manufacturing Violations
On March 24, 2017 the FDA issued a warning letter to The Sanapac Company, Inc.
Recalls & Warnings
October 04, 2016
Turmeric Spice Recalled Due to Elevated Lead Levels
On September 26, 2016, Spices USA Inc. issued a recall of 772 bags of TASTY SAWA GROUND TURMERIC because it contains elevated levels of lead.
Recalls & Warnings
November 08, 2016
Chinese Herbal Supplement With Lead Contamination Recalled
On November 7, 2016, Ton Shen Health issued a recall its Life Rising brand of Side Head Regulator TT tablets because they were found to contain elevated levels of lead for children under the age of 18.
Recalls & Warnings
November 29, 2016
Bentonite Clay Promoted for "Detoxification" Contaminated With Lead
On June 17, 2016, the FDA issued a warning letter to Best Bentonite, following a facility inspection and laboratory analysis of product sample which found the company's Best Bentonite to be contaminated with lead.
Recalls & Warnings
August 08, 2014
FDA Warns Puerto Rico Supplement Seller
On June 30, 2014, the FDA issued a warning letter to Mr. Ramon Rosa, following a review of his website, www.aceitedeguanabana.
Recalls & Warnings
January 13, 2019
Sexual Enhancement Supplement Recalled
On January 8, 2019, Happy Together, Inc. issued a recall of the sexual enhancement supplement Rhino 5k because FDA analysis found it to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
December 11, 2018
Sexual Enhancement Supplement Found to Contain Prescription Drug
On December 10, 2018, the FDA warned consumers that the sexual enhancement supplement On Demand contains undeclared sildenafil.
Recalls & Warnings
December 01, 2018
Sexual Enhancement Supplements Contain Prescription Drugs
The FDA recently warned consumers that the following sexual enhancement supplements were found to contain undeclared sildenafil and/or tadalafil. Use the links below to read the complete warning letter for each:
- Willy Go Wild
Recalls & Warnings
October 16, 2018
Weight Loss Supplement Containing Hidden Drug Recalled
On October 15, 2018, Fat Burners Zone issued a recall of one lot of its Zero Xtreme weight loss supplement because it was found to contain sibutramine.
Recalls & Warnings
January 22, 2019
FDA Warns Consumers Not to Use "The Silver Bullet"
On January 10, 2019, the FDA warned consumers not to buy or use the sexual enhancement supplement The Silver Bullet because it contains undeclared sildenafil and tadalafil.
Recalls & Warnings
March 06, 2019
FDA Warns Consumers Not to Use Certain Sexual Enhancement Supplements
On January 28, 2019, the FDA warned consumers not to buy or use the following sexual enhancement supplements were found to contain undeclared sildenafil and/or tadalafil.
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
April 12, 2019
CDC: Kratom Linked to 91 Deaths
Kratom may have played a role in twice as many deaths as has been previously estimated, according to a report issued today by the Center of Disease Control (CDC).
Recalls & Warnings
July 28, 2008
Seizure of Xiadafil VIP Tablets After Company Refuses Recall
On July 24, 2008, the FDA announced that U.S. Marshals seized nearly $74,000 worth of Xiadafil VIP tablets, Lots 6K029 and 6K209-SEI, distributed by SEI Pharmaceuticals, Inc. of Miami, Fla.
Recalls & Warnings
March 29, 2013
Prescription Drugs Found In Prostate and Sexual Enhancement Supplements
October 24, 2012 the FDA issued a warning letter to USA Far Ocean Group Inc./ Health & Beauty Group Inc. because a number of the company's dietary supplements were found to contain pharmaceutical drugs, or were promoted with statements that constitute drug claims.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
August 22, 2012
FDA Warns of Life-Threatening Side Effects and Withdrawal Symptoms from Two Arthritis, Muscle Pain Supplements
On August 21, 2012, the FDA issued a warning to consumers about the dangers of taking Reumofan Plus and Reumofan Plus Premium, two dietary supplements marketed for arthritis, osteoporosis and muscle pain.
Recalls & Warnings
September 13, 2014
Three Weight Loss Supplements Found to Contain Drugs
On September 12, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain drugs.
Recalls & Warnings
October 17, 2012
Herbal Supplement Company Warned For Manufacturing Violations and Drug Claims
On October 2, 2012, the FDA issued a warning letter to Amazing Herbs Nutraceuticals following a facility inspection which found violations of Current Good Manufacturing Practices (CGMPs) for dietary supplements.
Recalls & Warnings
August 01, 2013
Maker of Algae Products Warned For Drug Claims, Manufacturing Violations
On March 8, 2013, the FDA issued a warning letter to Algaen Corporation following a facility inspection and website review which found statements made about the company's AlgaBerry products to be drug claims.
Recalls & Warnings
February 15, 2013
FDA Seizes Weight Loss Supplements Containing Undeclared Drug
On Feb. 14, 2013, U.S. Marshals acting on behalf of the FDA seized weight loss supplements from Globe All Wellness, LLC which were found to contain the undeclared drug sibutramine hydrochloride.
Recalls & Warnings
May 24, 2013
Seller of Liquid Minerals, Joint Care and Herbal Supplements Warned For Manufacturing Violations, Drug Claims
On April 8, 2013, the FDA issued a warning letter to Body Systems, Inc.
Recalls & Warnings
November 07, 2013
FDA Warns Consumers of Weight Loss Supplement Containing Multiple Drugs
On November 7, 2013, the FDA warned consumers not to buy or use Jimpness Beauty Fat Loss Capsules because they were found to contain sibutramine, phenolphthalein, and sildenafil.
Recalls & Warnings
February 22, 2012
"Japan Weight Loss Blue" Pills Dangerous According to FDA
On February 18, 2012, the U.S. Food and Drug Administration (FDA) advised consumers not to purchase or use “Japan Weight Loss Blue,” a product for weight loss sold on various websites, including www.vitaminbestbuy.com.
Recalls & Warnings
November 06, 2009
FDA Warns that "Stiff Nights" Enhancement Supplement Contains Undeclared Drug
On November 5, 2009 the U.S. Food and Drug Administration (FDA) warned consumers that Stiff Nights, a product marketed as a dietary supplement for sexual enhancement, contains an ingredient that can dangerously lower blood pressure and is illegal.
Recalls & Warnings
February 26, 2015
Weight Loss Supplement Found to Contain Multiple Drugs
On February 25, 2015, the FDA warned consumers not to buy or use weight loss supplement Lean Body Extreme because it was found to contain sibutramine, desmethyl sibutramine, phenolphthalein, and sildenafil.
Recalls & Warnings
October 24, 2015
Four Weight Loss Supplements Found To Contain Drugs
On October 23, 2015, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine, phenolphthalein and/or sildenfil. Each was identified during an examination of international mail shipments:
Recalls & Warnings
March 13, 2015
Weight Loss Supplement Contains Prescription Anti-Depressant, Other Drugs
On March 3, 2015, the FDA warned consumers not to buy or use weight loss supplement Natural Max Slimming because it was found to contain fluoxetine, sildenafil and sibutramine.
Recalls & Warnings
May 02, 2016
Weight Loss Supplements Containing Hidden Drugs Recalled
On April 29, 2016 Making It A Lifestyle, L.L.C. issued a recall of all lots of weight loss supplements 3rd Degree, Black Gold X Advanced and Black Label X because they were found to contain undeclared sibutramine and sildenafil.
Recalls & Warnings
April 08, 2016
Large Doses of Stimulant Methylsynephrine Found In Weight Loss Supplements
Amounts of methylsynephrine found in some supplements exceed prescription dosages, according to new study published in Drug Testing and Analysis.
Recalls & Warnings
March 20, 2016
FDA Warns of Weight Supplements Containing Undeclared Drugs
On March 17, 2016, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared drugs:
Recalls & Warnings
February 02, 2018
Seller of Vitamin D, Omega-3's, Whey Protein & More Warned for Drug Claims
On January 24, 2018 the FDA issued a warning letter to Young Health Products, LLC following a review of the company's website which found promotional statements and testimonials made about the certain products, including Lugol's Solution 2%, Liquid Vitamin D3, Probimune, Flax Seed & Omega ...
Recalls & Warnings
April 12, 2019
Sexual Enhancement Supplement for Men Recalled
On April 9, 2019, SD Import, LLC issued a recall of Aphrodisiac, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
April 08, 2019
Herbal "Coffee" for Sexual Enhancement Recalled
On March 22, 2019, Brian Richardson DBA "In Tha Pink" issued a recall of ground Kopi Jantan Tradisional [sic] Natural Herbs Coffee, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
March 06, 2019
GoLean Detox Recalled
On February 25, 2019, GoLean Detox USA issued a recall of all lots of GoLean DETOX capsules within expiry because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
January 22, 2019
Weight Loss Supplement Tainted With Drug
On January 10, 2019, the FDA warned consumers not to use the weight loss supplement Slimina, because it was found to contain sibutramine.
Recalls & Warnings
March 26, 2019
LEOPARD Miracle Honey Recalled
On March 22, 2019, USA LESS issued a recall of all lots of LEOPARD Miracle Honey, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
March 23, 2019
BLUEFUSION Capsules for Sexual Enhancement Recalled
On March 21, 2019, Ata Int. Inc. issued a recall of its BLUEFUSION capsules, which are promoted for sexual enhancement, because FDA analysis found them to contain sildenafil, tadalafil, desmethyl carbodenafil, dithiodesmethyl carbodenafil, scutellarin and daidzein.
Recalls & Warnings
May 19, 2018
Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On May 17, 2018, Shoreside Enterprises, Inc., recalled certain lots of its sexual enhancement supplements 7K and Poseidon 4500 (Extreme 1000 mg) because they were found to contain undeclared sildenafil and/or tadalafil.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
September 04, 2018
Federal Court Shuts Down Maker of Sexual Enhancement Products
On August 30, 2018, the FDA announced that U.S.
Recalls & Warnings
August 01, 2013
Fat Loss and Muscle Enhancement Supplements Found To Contain DMAA
On June 17, 2013, the FDA issued a warning letter to Formulife, Inc., dba Purus Labs, Inc., following a facility inspection which found the dietary supplements Fat Smack XR Thermolipolytic, Muscle Marinade Fresh Fruit and Muscle Marinade Cherry Limeade to contain DMAA.
Recalls & Warnings
December 22, 2008
FDA Warns Consumers About Tainted Weight Loss Pills
On December 22, 2008 the U.S. FDA alerted consumers not to purchase or consume any of more than 25 different products marketed for weight loss because they contain undeclared, active pharmaceutical ingredients that may put consumers’ health at risk.
Recalls & Warnings
November 25, 2007
Recall of Men's "Sexual Energy" Supplement Expanded to Include "Energy Max" Product
On November 16, 2007, American True Man Health Incorporated announced that it is expanding it's voluntary recall of the Company's dietary supplement product sold under the name and identified as True Man's Sexual Energy Nutriment Men's Formula with the expiration date up to and including December ...
Recalls & Warnings
April 04, 2013
Three More Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On April 3, 2013, the FDA warned consumers that the sexual enhancement supplements AFFIRM XL, Love Rider and Ninja Mojo were found to contain undeclared erectile dysfunction drugs. Consumers are urged to stop using these supplements immediately.
Recalls & Warnings
March 13, 2015
Three Weight Loss Supplements Found to Contain Drugs
The FDA recently warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
August 07, 2015
Male Enhancement and Weight Loss Supplement Containing Drugs Recalled
On August 6, 2015 Blue Square Market Inc. issued a recall of the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
August 15, 2017
Protein Supplements Can Be Dangerous in People with Rare Genetic Disorder
It was recently reported by Yahoo Australian News (August 14, 2017) that a 25-year-old woman died after taking protein supplements and eating protein-rich foods such as lean meats and egg whites in preparation for a fitness competition.
Recalls & Warnings
August 03, 2017
AMPT "Sexual Enhancement" Coffee Recalled
On August 1, 2017, AMPT Life, LLC, recalled all lots of AMPT Coffee after FDA testing found sildenafil and tadalafil.
Recalls & Warnings
September 23, 2017
Enhancement Supplement "Vegetable Vigra" Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled VEGETABLE VIGRA [sic] after FDA testing found undeclared presence of sildenafil.
Recalls & Warnings
September 21, 2017
Sexual Enhancement Supplements Containing Prescription Drugs Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled the following supplements because they were found to contain undeclared sildenafil and tadalafil:
- Rhino 7 Platinum 5000 capsules, Lot# R7-D5K1011H
Recalls & Warnings
April 17, 2018
Sexual Enhancement Supplement Recalled
On April 16, 2018, Epic Products, LLC, recalled its sexual enhancement supplement Euphoric because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
April 14, 2018
Men's Sexual Enhancement Supplement Recalled
On April 12, 2018, AMA Wholesale Inc. recalled its sexual enhancement supplement Rhino 69 Extreme 50000 because it was found to contain undeclared tadalafil.
Recalls & Warnings
February 27, 2018
Weight Loss Supplement Containing Undeclared Drug Recalled
On February 27, 2018, Bella All Natural recalled their "Diet Capsules" because they contain sibutramine.
Recalls & Warnings
May 17, 2016
Sexual Enhancement Supplement Contains Undeclared Drugs
On May 6, 2016, the FDA issued a warning letter to Economax, LLC because the company's sexual enhancement supplement, Super Power Khan was found to contain sildenafil and hydroxyhomosildenafil.
Recalls & Warnings
July 18, 2017
Hidden Drugs Found in Sexual Enhancement Products
On July 17, 2017, the FDA posted public notifications after finding multiple sexual enhancement products contained hidden drug ingredients.
Recalls & Warnings
July 18, 2017
Herbal "Sexual Enhancement Coffee" Recalled
On July 13, 2017, Bestherbs Coffee LLC recalled their product New Kopi Jantan Tradisional Natural Herbs Coffee after FDA testing found desmethyl cabodenafil.
Recalls & Warnings
July 26, 2017
Weight Loss Supplements Containing Undeclared Drug Recalled
On July 25, 2017, EZ Weight Loss TX recalled their supplements La Bri's Body Health Atomic and Xplode capsules after FDA analysis found the products to be tainted with sibutramine.
Recalls & Warnings
July 22, 2017
Sexual Enhancement Supplement Containing Prescription Drugs Recalled
On July 21, 2017, Ultra Shop Supplement recalled Super Panther 7K capsules after FDA testing found them to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
June 25, 2014
More Generic Versions of Heart Medication Recalled
As reported in the New York Times on June 24, 2014, certain lots of the blood pressure medication metoprolol succinate extended-release have recently been recalled. The recalling manufacturers are the Indian companies Dr. Reddy's Laboratories and Wockhardt.
Recalls & Warnings
November 28, 2008
Fat Loss Supplement Recalled for Containing Prescription Drug
On November 26, 2008, the FDA reported that Zhen De Shou Fat Loss Capsules are being recalled by the distributor, Fashion Sanctuary. FDA analysis found the product to contain undeclared sibutramine, an FDA approved drug used as an appetite suppressant for weight loss.
Recalls & Warnings
April 23, 2009
Recall of "Slimming" Supplements Spiked with Drug
On April 20, 2009 the U.S. FDA reported a nationwide recall of dietary supplements distributed by Universal ABC Beauty Supply International.
Recalls & Warnings
September 13, 2010
Recall of Herbal Slimming Supplement Spiked with Drug
The U.S. FDA posted a notice dated August 18, 2010 indicating that Slim-30 Herbal Supplement (Lot 6032101) has been recalled by its distributor, J & H Besta Corp.
Recalls & Warnings
February 01, 2012
FDA Warns Seller of Almased Weight Loss Product of Marketing Violations
On January 18, 2011, the U.S. FDA sent a Warning Letter to Almased USA regarding legal violations in its marketing of its Almased product (Almased Synergy Diet).
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
Recalls & Warnings
November 08, 2012
FDA Warns Consumers of "Slimming Coffee"
On November 8, 2012, the FDA warned consumers not to purchase or use Best Share Green Coffee: Brazilian Slimming Coffee after FDA testing revealed the coffee, which was promoted for weight loss, contains the drug sibutramine.
Recalls & Warnings
March 13, 2018
FDA Warns Seller of Milk Thistle, Chromium, Joint Supplements & More for Manufacturing Violations
On March 9, 2018, the FDA issued a warning letter to Carol Bond Health Foods following a facility inspection which found a number of the company's products, including Dolomite, Lipo Fat Fighter, ThistleX, Injuv, and Vinpocentine, to be ...
Recalls & Warnings
September 12, 2012
Distribution of Weight Loss Product Containing DMAA Should Be Stopped Immediately, Warns FDA
On August 28, 2012, the FDA issued a warning letter to Regeneca stating that the company's RegeneSlim, which was promoted as a dietary supplement for weight loss, is adulterated with dimethylamylamine (DMAA) and that failure to immediately cease distribution of this product could result in FDA ...
Recalls & Warnings
November 20, 2013
$2 Million In Weight Loss and "Fat-Burning" Supplements Seized
On November 12, 2013, U.S. Marshals seized more than $2 million worth of supplements from Georgia-based Hi-Tech Pharmaceuticals, Inc.
Recalls & Warnings
September 27, 2013
Company Warned For Distributing Weight Loss Supplement Containing DMAA, Drug Claims
On September 6, 2013, the FDA issued a warning letter to Pure Energy Products, Inc., following a facility inspection which found that the company distributes a weight loss supplement, called obestrim, which contains dimethylamylamine, or DMAA.
Recalls & Warnings
January 31, 2014
Weight Loss Supplement Containing DMAA Recalled
On January 30, 2014, YoungYou International ("YoungYou") issued a voluntary recall of Mega Slim Herbal Appetite Management capsules because they were found to contain DMAA.
Recalls & Warnings
September 15, 2013
Creatine Powder Containing DMAA Recalled
On September 12, 2013, Ge Pharma, LLC issued a recall of grape and fruit punch flavors of Creafuse Powder because they contain 1,3 dimethylamylamine (DMAA).
Recalls & Warnings
July 24, 2013
USPLabs Destroys $8 Million Worth of Supplements Containing DMAA
On July 16, 2013, the FDA announced that USPLabs voluntarily destroyed its inventory of OxyElite Pro and Jack3d, workout supplements that contain DMAA. DMAA has been linked to a number of adverse effects and is not allowed to be sold as a dietary supplement ingredient.
Recalls & Warnings
June 23, 2013
Weight Loss Supplements Containing DMAA Recalled
On June 15, 2013, dietary supplement retailor Beta Labs, LTD issued a recall of certain lots of "fat burner" and weight loss supplements Oxyphen XR, Phentalene, Phen FX and Red Vipers because they contain 1,3-dimethylamylamine (DMAA).
Recalls & Warnings
July 20, 2017
Jail Time for Seller of Spiked Supplements
On July 18, 2017, Derek Vest, the former president of Gentech Pharmaceutical located in Fort Meyers, Florida, was sentenced to 18 months in prison for introducing misbranded food into interstate commerce.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
November 25, 2016
DMAA Product Recalled
On November 23, 2016, NutriVitaShop (a dba of Naturecom Inc.) issued a recall of its DMAA net weight 500g because it may contain DMAA.
Recalls & Warnings
February 09, 2017
Federal Court Orders Dietary Supplement Distributor to Stop Selling Its Products
On February 9, 2017, the FDA announced that VivaCeuticals Inc, doing business as Regeneca Worldwide, has been ordered by a federal court to stop selling its products, which were found to contain unsafe ingredients including DMAA.
Recalls & Warnings
October 10, 2014
Weight Loss Supplement Found to Contain Multiple Drugs
On October 10, 2014, the FDA warned consumers not to purchase or use the dietary supplement Japan Hokkaido Slimming Weight Loss Pills because it was found to multiple contain undeclared drugs, including sibutramine, phenolphthalein, benzocaine and diclofenac.
Recalls & Warnings
September 02, 2014
Recall of Weight Loss Supplement Expanded
On August 27, 2014, Regeneca Worldwide expanded its previous recall of two lots of appetite control supplement RegenESlim to include two more lots because FDA tests found them to contain DMAA (1,3-dimethylamylamine).
Recalls & Warnings
August 08, 2014
Weight Loss Supplement Found to Contain DMAA Recalled
On August 6, 2014, Regeneca Worldwide issued a recall of two lots of appetite control supplement RegenESlim because FDA tests found them to contain DMAA (1,3-dimethylamylamine).
Recalls & Warnings
October 27, 2004
Some Supplements Can Damage Eyes At High Doses and with Topical Use
As reported by Reuters Health on October 21, 2004, an article in the current issue of the American Journal of Ophthalmology suggests that some herbal remedies and nutritional supplements can damage the eyes.
Recalls & Warnings
August 11, 2008
Stroke Attributed to Xenadrine Ephedra-Free Supplement
A case report in the journal Military Medicine (July 2008) attributes the occurance of vasospasm and stroke in a 36 year-old women to use of Xenadrine-EFX, a supplement used for weight loss.
Recalls & Warnings
September 27, 2016
Seller of B Vitamins, Omega-3s and More Warned for Manufacturing Violations, Drug Claims
On September 15, 2016, the FDA issued a warning letter to Positive Power Nutrition, following a facility inspection which found the company's products, including High Energy C-Complex, Positive Vitality, Positive Performance, Positive CardioGuard, B-Complex 100, Positive Essentials, Positive ...
Recalls & Warnings
September 02, 2016
Kratom To Be Classified a Schedule I Controlled Substance
On August 30, 2016, the U.S. Drug Enforcement Agency (DEA) announced its intent to classify the active compounds (mitragynine and 7-hydroxymitragynine) in kratom as Schedule I controlled substances.
Recalls & Warnings
April 11, 2013
Glutathione Supplement Maker Warned For Manufacturing Violations and Drug Claims
On April 5, 2013, the FDA issued a warning letter to The Glutathione Corporation following a facility inspection which found the company's supplements, including Ultrathione 500 Glutathione, Ultrathione 1000 Sports Pack, Ultrathione Performance, Ultrathione Extreme Performance, Ultrathione Health ...
Recalls & Warnings
February 06, 2018
FDA Links 44 Deaths to Kratom
On February 6, 2018, FDA announced it is now aware of 44 deaths associated with the use of kratom, an herb often promoted for pain relief, energy and for treating opioid withdrawal symptoms. This is an increase from the 36 deaths associated with kratom that the agency reported in November 2017.
Recalls & Warnings
November 14, 2017
36 Deaths Associated With the Use of Kratom Products
On November 14, 2017, FDA Commissioner Scott Gottlieb, M.D. issued a statement on the dangers of using products containing kratom, an herb often promoted for pain relief, energy and for treating opioid withdrawal symptoms.
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
July 18, 2017
Seller of "Super Strength Prostate Formula," Acai Berry Supplements & More Warned for Drug Claims
On June 30, 2017, the FDA issued a warning letter to Nature's Health Company, LLC, following a facility inspection and review of the company's website, www.natureshealthcompany.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...
Recalls & Warnings
August 02, 2014
Judge Orders BioAnue To Stop Illegal Cancer and Disease Claims
On July 23, 2014, Gloria D.
Recalls & Warnings
February 01, 2013
DMAA Supplement Linked to Runner's Death
The cause of death of London marathon runner Claire Squires has been ruled by the investigating coroner as cardiac failure due to extreme exertion, complicated by DMAA toxicity.
Recalls & Warnings
April 29, 2012
FDA Says DMAA Can't Be Sold as a Supplement -- Warns Sellers
On April 27, 2012, the U.S.
Recalls & Warnings
August 08, 2017
Study Shows Severe Risk in Drinking Hydrogen Peroxide
An analysis of records from the National Poison Data System from a 10-year period ending in 2011 identified 294 reported incidents of people developing adverse symptoms after ingesting 35% (high-concentration) hydrogen peroxide (H202). Among these, 41 (13.
Recalls & Warnings
October 07, 2017
Weight Loss Supplement Recalled
On October 5, 2017, Kiriko, LLC recalled recalled all lots of A1 Slim 30 capsules after FDA analysis found the products to contain sibutramine, phenolphthalein and N-Desmethyl sibutramine.
Recalls & Warnings
December 19, 2017
Blue Pearl All Natural Enhancement Supplement Recalled
On December 13, 2017, Marmex Corp. recalled all lots of its sexual enhancement supplements Blue Pearl All Natural Male Enhancement Supplement, 500mg because they were found to contain undeclared sildenafil.
Recalls & Warnings
December 01, 2017
Nutra Labs Recalls Sexual Enhancement Supplements Containing Undeclared Drugs
On November 30, 2017, Nutra Labs Inc. recalled certain lots of its sexual enhancement supplements Bull 1800 mg Capsules and Chao Jimengnan 150 mg Tablets because they were found to contain undeclared sildenafil.
Recalls & Warnings
November 27, 2011
FDA Seeks Permanent Injunction Against Dietary Supplement Maker -- 400+ Products Affected
On November 23, 2011, the U.S. FDA took legal action against a dietary supplement maker and owner for substituting ingredients and products without noting the changes on the final product labels. The permanent injunction, filed on behalf of the FDA by the U.S.
Recalls & Warnings
July 19, 2011
FDA Warns of Unsafe Drug in Slimming Supplements
On July 8, 2011, the U.S. FDA advised consumers not to purchase or use Slim Forte Slimming Capsule and Slim Forte Double Power Slimming Capsules. FDA laboratory analysis confirmed that these products contain sibutramine. Sibutramine is a controlled substance that was removed from the U.S.
Recalls & Warnings
November 21, 2010
FDA Warns Consumers Not to Use Vigor-25
On November 19, 2010, the U.S.
Recalls & Warnings
January 04, 2011
Weight Loss Supplement Containing a Drug Can Cause Serious Adverse Events, Warns FDA
On December 31, 2010 the U.S. FDA warned consumers not to use Fruta Planta weight loss products because they contain sibutramine, a drug withdrawn from the market in December 2010 for safety reasons.
Recalls & Warnings
February 25, 2011
Weight Supplement Found by FDA to Contain Prescription Drug
The U.S. FDA posted an announcement (dated February 9, 2011) by Svelte 30 Nutritional Consultants indicating that a sample of Svelte 30 orange & gray capsule was collected and tested by FDA in January 2011.
Recalls & Warnings
November 15, 2009
Nationwide Recall of a Weight Loss Supplement Found to Contain Undeclared Drug Ingredients
On November 12, 2009, The U.S. Food and Drug Administration (FDA) announced that it informed GMP Herbal Products, Inc. that its product "Pai You Guo," a weight loss dietary supplement, contains undeclared drug ingredients.
Recalls & Warnings
July 23, 2009
Weight Loss Supplements Found to Contain Prescription Drug
On July 15, 2009, the U.S. FDA announced that Young You Corporation was recalling four weight loss supplements that contain an undeclared drug ingredient.
Recalls & Warnings
July 19, 2010
FDA Warns of Drug in Herbal Slimming Supplement
On July 19, 2010, the U.S. FDA issued a safety warning to consumers regarding Slim-30 Herb Supplement. FDA analysis of the product found it to contain undeclared N-Desmethyl Sibutramine and traces of Sibutramine. Sibutramine is an FDA-approved drug used as an appetite suppressant for weight loss.
Recalls & Warnings
October 24, 2012
FDA Warns Consumers of Weight Loss and Body Reshaping Supplements Containing Controlled Substance
On October 24, 2012, the FDA warned consumers not to purchase or use weight loss and body reshaping dietary supplement Zi Xiu Tang Bee Pollen Capsules (also referred to as Zi Xiu Tang Beauty, Face & Figure Capsules) because this product was found to contain sibutramine.
Recalls & Warnings
August 09, 2012
Makers of Mineral Water Containing Lithium Warned
On July 20, 2012, the FDA issued a warning letter to Lithia Mineral Water, Inc. declaring Lithia mineral water product to be an approved drug following a 2011 facility inspection.
Recalls & Warnings
July 26, 2012
Sexual Enhancement Supplements Containing Prescription Drug Recalled
On July 20, 2012, CRM Laboratories issued a voluntary recall of all X-Rock 3 Day Pill For Men and Z-Rock All Natural Male Supplements after FDA testing found these products contain the drugs sildenafil and hydroxythiohomosildenafil.
Recalls & Warnings
November 06, 2012
Recall of "Bee Pollen" Supplement Found to Contain Drug
On October 24, 2012, Zi Xiu Tang Success, LLC issued a voluntary recall of Classic Zi Xiu Tang Bee Pollen Capsules and Ultimate Formula Capsules because the supplements contain the undeclared drug sibutramine.
Recalls & Warnings
November 09, 2012
FDA Warns Consumers of Two Weight Loss Supplements Containing a Drug
On November 8, 2012, the FDA warned consumers not to buy or use weight loss supplements Beautiful Body Slim and Japan Hokkaido Slimming Weight Loss Pills after FDA testing found these products contain the drug sibutramine.
Recalls & Warnings
September 21, 2012
Sexual Enhancement Supplements For Men and Women Recalled Due to Prescription Drug Risk
On August 23, 2012, Evol Nutrition Associates Inc./Red Dawn issued a voluntary recall of Mojo Nights after FDA testing revealed the male sexual enhancement supplement contains undeclared prescription drugs tadalafil and sildenafil.
Recalls & Warnings
September 18, 2012
Men’s Sexual Enhancement Supplement Containing Prescription Drug Recalled
On September 12, 2012, Body Basics Inc. issued a voluntary nationwide recall of ACTRA-Sx 500 Dietary Supplement Capsules after independent laboratory testing confirmed the supplement contains the prescription drug sildenafil citrate.
Recalls & Warnings
January 28, 2013
Recall: Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 24, 2013, D&S Herbals, LLC, dba Freedom Trading, announced a voluntary recall of one lot of the male sexual enhancement dietary supplement Super Power because it was found to contain undeclared sildenafil.
Recalls & Warnings
January 24, 2013
Excessive Lead and Mercury Found In Chinese Herbal Supplements
On January 23, 2013, Health Canada warned consumers that certain batches of the Chinese dietary supplements [W.S.
Recalls & Warnings
December 19, 2012
Sexual Enhancement Supplements Recalled Due To Undeclared Drugs
On December 17, 2012, Performance Plus Marketing, Inc.
Recalls & Warnings
January 24, 2013
"Thermo Stimulating" Supplement Recalled -- Contained DMAA
On January 23, 2013, Health Canada advised consumers not to use certain batches of Muscletech Hydroxystim capsules which were found to contain DMAA (1,3-dimethylamylamine) and have been recalled by their distributor.
Recalls & Warnings
January 23, 2013
USPLabs Settles Class Action Lawsuit Over Controversial DMAA Ingredient
On December 18, 2012, a settlement was approved in the class action lawsuit against USPLabs, which alleged the company's use of the ingredient DMAA (1,3-dimethylamine) in the supplements OxyELITEPRO and Jacked3d was not safe or legal.
Recalls & Warnings
January 18, 2013
Recall: Iron Supplement Containing Motion Sickness Drug
On January 18, 2012, Advance Pharmaceutical Inc. announced the recall of one lot of Rugby NATURAL IRON SUPPLEMENT Ferrous Sulfate Tablets 325 mg, because the bottles were found to contain meclizine HCl 25 mg tablets instead.
Recalls & Warnings
December 21, 2012
Weight Loss Supplement Containing Undeclared Drug Recalled
On December 19, 2012, P&J Trading issued a voluntary recall of SLIMDIA REVOLUTION after FDA testing found the dietary supplement contains undeclared sibutramine.
Recalls & Warnings
March 21, 2013
FDA Warns Consumers: Three Sexual Enhancement Supplements Found To Contain Drugs
On March 21, 2013, the FDA warned consumers not to purchase or use three sexual enhancement dietary supplements, Rock-It Man, Libido Sexual Enhancer, and Stiff Days, because they were found to contain sildenafil or sildenafil analogues.
Recalls & Warnings
March 13, 2013
Recall: Male Sexual Enhancement Supplement Containing Drugs
On March 12, 2013, Green Planet, Inc. issued a recall of one lot of the male sexual performance enhancer supplement Night Bullet because it was found to contain trace amounts of sulfohydroxyhomosildenafil and aminotadalafil, analogues of the prescription drug sildenafil.
Recalls & Warnings
February 25, 2013
Weight Loss Supplement Recalled Due To Undeclared Drug
On February 21, 2013, Olaax Corp. issued a voluntary, nationwide recall of the company's weight loss supplement MAXILOSS Weight Advanced softgels because they were found to contain undeclared Sibutramine.
Recalls & Warnings
May 08, 2013
Three Sexual Enhancement Supplements Recalled for Undeclared Drug Risk
On May 7, 2013, BeaMonstar Products issued a voluntary recall of sexual enhancement supplements SexVoltz and Velextra because they were found to contain an undeclared drug.
Recalls & Warnings
May 03, 2013
Recall: Two Sexual Enhancement Supplements Containing Undeclared Drugs
On May 1, 2013, American Lifestyle issued a voluntary recall of all lots of sexual enhancement supplements Vicerex and Black Ant because they were found to contain undeclared prescription drugs.
Recalls & Warnings
April 25, 2013
FDA Warns Consumers of Sexual Enhancement Supplements Containing Drugs
On April 25, 2012, the FDA issued a warning to consumers, urging them not to purchase or use two sexual enhancement products which were found to contain undeclared drugs.
Recalls & Warnings
April 12, 2013
FDA Warns Consumers About The Dangers Of DMAA
On April 11, 2013, the FDA warned consumers about the dangers of dietary supplements containing the ingredient 1,3-dimethylamylamine (DMAA).
Recalls & Warnings
April 10, 2013
Recall: Sexual Enhancement Supplement Containing Undeclared Drug
On April 1, 2013, Consumer Concepts, Inc. issued a voluntary nationwide recall of ROCK-It MAN Male Enhancement Capsules, a dietary supplement promoted for sexual enhancement which was found to contain undeclared hydroxythiohomosildenafil, an analogue of the prescription drug sildenafil.
Recalls & Warnings
April 04, 2013
FDA Warns Consumers Of Weight Loss Supplement Containing Undeclared Drug
On April 4, 2013, the FDA warned consumers not to purchase or use the dietary supplement MAXILOSS Weight Advanced Blue because it was found to contain sibutramine.
Recalls & Warnings
June 19, 2013
Weight Loss Supplements Found To Contain Undeclared Drugs, FDA Warns
On June 17, 2013, the FDA advised consumers not to purchase or use the weight loss supplements listed because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein.
Recalls & Warnings
June 19, 2013
Sexual Enhancement Supplement Found To Contain Undeclared Drug
On June 17, 2013, the FDA advised consumers not to purchase or use sexual enhancement supplement Royal Dragon Herbal Tonic Balls because it was found to contain undeclared vardenafil.
Recalls & Warnings
June 14, 2013
Weight Loss Supplement Found To Contain Undeclared Drug
On June 6, 2013, the FDA warned consumers that weight loss dietary supplement XIYOUJI QINGZHI CAPSULE was found to contain undeclared simbutrimine.
Recalls & Warnings
June 13, 2013
FDA Warns Consumers of Sexual Enhancement Supplements Containing Prescription Drugs
On June 10, 2013, the FDA warned consumers that a number of sexual enhancement supplements have been found to contain undeclared drugs such as sildenafil and tadalafil.
Recalls & Warnings
June 12, 2013
Weight Loss Supplement Recalled Due To Undeclared Drugs
On June 11, 2013, Bethel Nutritional Consulting, Inc. issued a voluntary recall of one lot of Bethel 30 herbal weight loss supplement because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
September 05, 2013
Seller of Herbal Supplements and "Tonics" Warned For Manufacturing Violations and Drug Claims
On May 24, 2013, the FDA issued a warning letter to Sundial Herbal Products following a facility inspection which found the company's products, including Woman Back Tonic, Koromantee, Wood and Root Tonic, Arthritis, Asthma, Diabetics, Sundial Ashanti Weight Loss Energy Lifter, Appetite Suppresser, ...
Recalls & Warnings
September 03, 2013
Sexual Enhancement Supplements Containing Undeclared Drug Recalled
On August 27, 2013, Hardmenstore.com issued a voluntary recall of sexual enhancement supplements 72HP, Evil Root and Pro Power Max because they were found to contain undeclared sildenafil.
Recalls & Warnings
August 23, 2013
Sexual Enhancement Supplement Containing Undeclared Drugs Recalled
On August 12, 2013, Jack Rabbit Inc. issued a voluntary recall of one lot of sexual enhancement supplement Jack Rabbit because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
August 08, 2013
Weight Loss Supplement Containing Undeclared Drug Recalled
On August 3, 2013, CTV Best Group issued a voluntary recall of all lots of weight loss supplement BEST SLIM because it was found to contain sibutramine.
Recalls & Warnings
August 06, 2013
Weight Loss Supplement Recall Expanded
On August 5, 2013, Bethel Nutritional Consulting, Inc., issued a voluntary recall of herbal weight loss supplements Quick Thin and Bethel Advance because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
July 23, 2013
Weight Loss Supplements Containing Undeclared Drugs Recalled
On July 19, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss supplements Esbelin siloutte te and Esbelin siloutte Herbal Blend with L-Carnitine because they were found to contain undeclared sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
July 22, 2013
Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On July 18, 2013, Volcano Company issued a voluntary recall all lots of Volcano Male Enhancement Liquid and Volcano Male Enhancement Capsules because they were found to contain undeclared desmethyl carbodenafil, dimethylsildenafil, and dapoxetine.
Recalls & Warnings
July 15, 2013
Two Sexual Enhancement Supplements Recalled
On July 5, 2013, Hardmenstore.com issued a voluntary recall of 430 lots of sexual enhancement supplements Silver Sword and Clalis because they were found to contain undeclared sildenafil.
Recalls & Warnings
June 30, 2013
Weight Loss Supplements Recalled Due To Undeclared Drug
On May 21, 2013, the Dolphin Intertrade Corp. issued a voluntary recall of one lot of weight loss supplement JaDera and all lots of weight loss supplement Xiyouji Qingzhi, which were found to contain the undeclared drug sibutramine.
Recalls & Warnings
June 28, 2013
Three Sexual Enhancement Supplements Found Contain Undeclared Drugs
On June 27, 2013, the FDA warned consumers not to buy or use MVP Mega, Exten 1300 and MaxTreme Zen, dietary supplements for sexual enhancement which were found to contain undeclared tadalafil or a combination of tadalafil and sildenafil.
Recalls & Warnings
June 27, 2013
Two Weight Loss Supplements Found To Contain Undeclared Drugs
On June 27, 2013, the FDA warned consumers not to use or buy two dietary supplements for weight loss which were found to contain the undeclared sibutramine and/or phenolphthalein.
Recalls & Warnings
June 27, 2013
Another Sexual Enhancement Supplement Found Contain Undeclared Drug
On June 27, 2013, the FDA warned consumers not to buy or use Silver sword, a dietary supplement for sexual enhancement which was found to contain undeclared sildenafil.
Recalls & Warnings
January 30, 2014
Four Weight Loss Supplements Found To Contain Drugs
On January 28, 2014, the FDA warned consumers not to buy or use the four weight loss supplements listed below because they were found to contain sibutramine or phenolphthalein.
Recalls & Warnings
January 23, 2014
"Slimming" Supplements Found to Contain Drugs
On January 21, 2014, the FDA advised consumers not to buy or use weight loss supplements Dream Body Slimming Capsule and Magic Slim because they were found to contain sibutramine and/or phenolphthalein.
Recalls & Warnings
January 14, 2014
Seven Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On January 9, 2014, Midwest Wholesale issued a voluntary recall of seven sexual enhancement supplements: Boost Ultra, XZone Gold, Sexy Monkey, Triple MiracleZen Platinum, Magic for Men, "New" Extenze, and New XZen Platinum.
Recalls & Warnings
December 26, 2013
Weight Loss Supplement Recalled
On December 23, 2013, Deseo Rebajar Inc. issued a voluntary recall of one lot of Burn 7 Capsules because they were found to contain sibutramine.
Recalls & Warnings
December 24, 2013
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On December 19, 2013, the FDA warned consumers not to buy or use weight loss supplement Dr. Ming's Chinese Capsule because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
December 20, 2013
Five Weight Loss Supplements Found to Contain Undeclared Drug
On December 19, 2013, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared sibutramine.
Recalls & Warnings
December 20, 2013
Two Sexual Enhancement Supplements Found to Contain Undeclared Drugs
On December 19, 2013, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared sildenafil or drugs chemically similar to sildenafil:
Recalls & Warnings
December 19, 2013
Seller of Sexual Enhancement Supplement and Tea Drink Warned for Drug Ingredients and Drug Claims
On December 5, 2013, the FDA issued a warning letter to Green Planet, Inc. following a facility inspection which found the company's sexual enhancement supplement Night Bullet to contain the drugs sulfohydroxyhomosildenafil, thioaildenafil and aminotadalafil.
Recalls & Warnings
November 21, 2013
Sexual Enhancement Supplement Found To Contain Multiple Drugs
On November 21, 2013, the FDA warned consumers not to buy or use sexual enhancement supplement Alpha Male because it was found to contain multiple undeclared drugs, including aminotadalafil, sulfosildenafil, sulfoaildenafil, hydroxythiohomosildenafil, dimethylsildenafil, and sildenafil.
Recalls & Warnings
November 21, 2013
FDA Warns of Five More Weight Loss Supplements Containing Undeclared Drugs
On November 21, 2013, the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein:
Recalls & Warnings
November 20, 2013
Three Sexual Enhancement Supplements Recalled
On November 16, 2013, Jobbers Wholesale issued a voluntary recall of sexual enhancement supplements Rhino 5 Plus, Maxtremezen, and Extenzone because they were found to contain the undeclared drugs desmethylcarbondenafil and dapoxetine.
Recalls & Warnings
November 20, 2013
Another Sexual Enhancement Supplement Recalled Due To Undeclared Drugs
On November 18, 2013, Fossil Fuel Products, LLC issued a voluntary recall of sexual enhancement supplement RezzRX because it was found to contain the undeclared drugs hydroxylthiohomosildenafil and aminotadalafil.
Recalls & Warnings
April 11, 2014
Two Weight Loss Supplements Found to Contain Drug
On April 10, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine.
Recalls & Warnings
April 03, 2014
Weight Loss Supplement Containing Drugs Recalled
On April 2, 2014, the FDA warned consumers not to buy or use weight loss supplement New You because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
March 31, 2014
Seven Sexual Enhancement Supplements Recalled Due to Undeclared Drugs
On March 27, 2014, Nova Products, Inc. issued a voluntary recall of sexual enhancement supplements African Black Ant, Black Ant, XZen Gold, ZXen Platinum, XZen 1200, XZone Gold and XZone 1200.
Recalls & Warnings
March 27, 2014
Bee Pollen and Weight Loss Supplements Recalled
On March 26, 2014, Pure Edge Nutrition, LLC issued a voluntary recall of Bella Vi Insane Bee Pollen Capsules, Bella Vi BTrim Ultimate Boost, Bella Vi BTrim Max, Bella Vi Extreme Accelerator, Bella Vi Insane Amp'd, and two lots of Bella Vi Amp'd Up because they were found to contain sibutramine, ...
Recalls & Warnings
March 27, 2014
Three Weight Loss Supplements Containing Drugs Recalled
On March 25, 2014, New Life Nutritional Center issued a voluntary recall of all lots of Super Fat Burner capsules, Maxi Gold capsules and Esmeralda softgels because they were found to contain sibutramine, phenolphthalein or a combination of both drugs.
Recalls & Warnings
March 19, 2014
Weight Loss "Coffee" Found to Contain Drug
On March 19, 2014, the FDA warned consumers not to buy or use Vitaccino Coffee because it contains sibutramine.
Recalls & Warnings
March 14, 2014
Arthritis and Muscle Pain Supplement Recalled
On March 12, 2014, Pain Free By Nature issued a recall of Reumofan Plus tablets sold on the company's website, www.painfreebynature.com, because it was found to contain the active pharmaceutical ingredients methocarbamol and diclofenac.
Recalls & Warnings
March 07, 2014
Sexual Enhancement Supplement Found to Contain Undeclared Drug
On March 7, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Weekend Warrior because it was found to contain undeclared thiosildenafil.
Recalls & Warnings
February 28, 2014
Weight Loss Supplement Contains Undeclared Drug
On February 18, 2014, Health Canada warned consumers not to use weight loss supplement LV Shou Reduces Fat because it contains sibutramine.
Recalls & Warnings
February 17, 2014
Weight Loss Supplement Recall Expanded to Include Additional Products
On February 4, 2014, MyNicKnaxs, LLC, which first issued a recall of Reduce Weight Fruta Planta, issued a recall of nine additional weight loss supplements: Magic Slim, Fruta Bio, SlimEasy, Super Fat Burning Bomb, Slim Xtreme, Meizi Evolution, Meizitang Strong Version Botanical Slimming, ...
Recalls & Warnings
September 27, 2013
Sexual Enhancement Supplement Containing Undeclared Drug Recalled
On September 24, 2013, the FDA warned consumers not to buy or use sexual enhancement supplement Wood-E because it was found to contain sildenafil. Sildenafil, the active ingredient in the prescription drug Viagra, is typically prescribed for erectile dysfunction.
Recalls & Warnings
April 18, 2014
Sexual Enhancement Supplement Contains Undeclared Drug
On April 16, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement S.W.A.G because it was found to contain sildenafil.
Recalls & Warnings
April 18, 2014
Weight Loss Supplement Recalled
On April 9, 2014, Nature's Universe issued a voluntary recall of all lots of Thinogenics weight loss capsules sold prior to February 6, 2014 because they were found to contain sibutramine.
Recalls & Warnings
November 06, 2013
Weight Loss Supplement Found to Contain Drugs
On November 5, 2013, the FDA advised consumers not to purchase or use the weight loss supplement Goodliness Fat-Reducing capsules, which were sold on various websites, because they were found to contain sibutramine and phenolphthalein.
Recalls & Warnings
October 30, 2013
Three Sexual Enhancement Supplements Found to Contain Prescription Drugs
On September 24, 2013, the FDA advised consumers not to purchase or use sexual enhancement supplements Xzen 1200, Xzen Gold and Xzen Xpress because they have been found to contain undeclared prescription drugs.
Recalls & Warnings
October 10, 2013
Six Weight Loss Supplements Found to Contain Drugs
On October 10, 2013, the FDA advised consumers not to purchase or use the six weight loss supplements listed below because they were found to contain one or both of the undeclared drugs sibutramine and phenolphthalein.
Recalls & Warnings
October 01, 2013
Three Sexual Enhancement Supplements Containing Undeclared Drug Recalled
On September 24, 2013, Haute Health, LLC issued a voluntary recall of all lots of sexual enhancement supplements Virilis Pro, PHUK and Prolifta, because they were found to contain sildenafil.
Recalls & Warnings
November 20, 2013
Two Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On November 19, 2013, Tendex issued a voluntary recall of one lot each of sexual enhancement supplements P-Boost and NatuRECT because they were found to contain tadalafil.
Recalls & Warnings
November 20, 2013
Weight Loss Supplement Found to Contain Drugs
On November 19, 2013, the FDA warned consumers not to buy or use weight loss supplement Slim Max because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
November 12, 2013
Weight Loss Supplement Found To Contain Drugs
On November 8, 2013, Health Canada warned consumers of a number of weight loss products that have been found to contain sibutramine or phenolphthalein, including Paiyouji Natural Slimming Capsules, which may be available for purchase to U.S. consumers through online retailers.
Recalls & Warnings
November 08, 2013
Sexual Enhancement Supplement Found To Contain Prescription Drugs
On November 8, 2013, the FDA warned consumers not to purchase or use sexual enhancement supplement Vitalikor Fast Acting because it was found to contain sildenafil and vardenafil.
Recalls & Warnings
June 20, 2014
FDA Warns Some Bee Pollen Products for Weight Loss Are Dangerous
On June 19, 2014, the FDA warned consumers that some weight loss products containing bee pollen have been found to contain hidden drugs.
Recalls & Warnings
June 18, 2014
Two Sexual Enhancement Supplements Found to Contain Drug
On June 17, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplements listed below because they were found to contain sildenafil.
Recalls & Warnings
June 18, 2014
Two Weight Loss Supplements Found to Contain Drugs
On June 17, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain drugs.
Recalls & Warnings
June 11, 2014
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On June 10, 2014, the FDA warned consumers not to buy or use weight loss supplement La Jiao Shou Shen because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
June 06, 2014
Sexual Enhancement Supplement Found to Contain Drug
On June 5, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement Zhen Gong Fu because it was found to contain sildenafil.
Recalls & Warnings
June 05, 2014
FDA Warns Consumers Not to Buy Use Weight Loss Supplement Containing Hidden Drugs
On June 5, 2014, the FDA warned consumers not to buy or use weight loss supplement B-Perfect because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
July 23, 2014
Sexual Enhancement Supplement Found to Contain Hidden Drug
On July 22, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement O.M.G. because it was found to contain sildenafil.
Recalls & Warnings
August 20, 2014
Sexual Enhancement Supplement Contains Hidden Drug
On August 11, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Arize because it was found to contain sulfoaildenafil. Sulfoaildenafil is structurally similar to sildenafil, the active ingredient in Viagra, which is prescribed for erectile dysfunction.
Recalls & Warnings
August 16, 2014
FDA Warns Consumers Not To Use Herbal Sexual Enhancement Supplement
On August 11, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Herbal Vigor Quick Fix because it was found to contain tadalafil. Tadalafil is the active ingredient in the prescription drug Cialis, which is prescribed for erectile dysfunction.
Recalls & Warnings
May 29, 2014
Recall of Sexual Enhancement Supplements Expanded
On May 28, 2014, Eugene Oregon, Inc. expanded its previous recall of certain lots of sexual enhancement supplements African Black Ant, Black Ant, and Mojo Risen. All lots of these supplements are now recalled.
Recalls & Warnings
May 16, 2014
Sexual Enhancement Supplement Contains Hidden Drug
On May 16, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement MV5 Days because it was found to contain sildenafil.
Recalls & Warnings
May 16, 2014
Weight Loss Supplement Found To Contain Drug
On May 16, 2014, the FDA warned consumers not to buy or use weight loss supplement Asset Bold because it was found to contain sibutramine.
Recalls & Warnings
May 15, 2014
FDA Warns Consumers Not To Buy Or Use Weight Loss Supplement
On May 12, 2014, the FDA warned consumers not to buy or use weight loss supplement Asset Bee Pollen because it was found to contain sibutramine.
Recalls & Warnings
May 07, 2014
Three Sexual Enhancement Supplements Recalled
On May 5, 2014 , Eugene Oregon, Inc. issued a precautionary, voluntary recall of sexual enhancement supplements African Black Ant, Black Ant and Mojo Risen.
Recalls & Warnings
May 06, 2014
Two Weight Loss Supplements Found To Contain Drug
On May 5, 2014, the FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain sibutramine.
Recalls & Warnings
May 01, 2014
Weight Loss Supplement Recalled
On April 29, 2014, dietary supplement distributor Bacai Inc. issued a voluntary recall of one lot of weight loss supplement LiteFit USA because it was found to contain sibutramine.
Recalls & Warnings
October 10, 2014
FDA Warns Consumers of Weight Loss Supplement Containing Undeclared Drugs
On October 10, 2014, the FDA warned consumers not to purchase or use the dietary supplement Sit and Slim II because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
November 05, 2014
Slimming Coffee Found to Contain Drug
On November 4, 2014, the FDA warned consumers not to purchase or use the dietary supplement V26 Slimming Coffee because it was found to contain sibutramine.
Recalls & Warnings
February 10, 2015
Five Weight Loss Supplements Recalled
On January 9, 2015, Detox Transforms Health and Nutrition issued a voluntary recall of five weight loss supplements because they were found to contain undeclared drugs:
Recalls & Warnings
January 31, 2015
Protein Powder Recalled Due to Food Poisoning Risk
On January 30, 2015, Aloha, Inc. issued a voluntary recall of all packages of Premium Protein powder blends in chocolate and vanilla because they have the potential to be contaminated with Staphylococcus enterotoxin .
Recalls & Warnings
January 24, 2015
FDA Warns Consumers Not To Use Sexual Enhancement Supplement
On January 21, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Happy Passengers, because it was found to contain undeclared sildenafil. Sildenafil, the active ingredient in Viagra, which is prescribed for erectile dysfunction.
Recalls & Warnings
January 06, 2015
FDA Warns of Weight Loss Supplement Dangers
On January 5, 2015, the FDA warned consumers that many weight loss products, including supplements, coffees and teas, may promise results they cannot deliver, or contain dangerous hidden drugs.
Recalls & Warnings
January 04, 2015
Sexual Enhancement Supplement Recalled
On December 30, 2014, Vivo Brand Management (Canada) issued a recall of one lot of the sexual enhancement supplement Forta for Men, because it was found to contain undeclared homosildenafil.
Recalls & Warnings
December 26, 2014
Three Sexual Enhancement Supplements Found to Contain Hidden Drugs
On December 23, 2014, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain an undeclared drug.
Recalls & Warnings
December 20, 2014
Weight Loss Supplement Recalled
On December 19, 2014, Bethel Nutritional Consulting, Inc. issued a recall of one lot of weight loss supplement SLIM-K Capsules because they were found to contain sibutramine.
Recalls & Warnings
November 26, 2014
Weight Loss Supplement Found to Contain Hidden Drug
On November 25, 2014, the FDA warned consumers not to buy or use weight loss supplement Slim Vie because it was found to contain sibutramine.
Recalls & Warnings
November 25, 2014
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement
On November 24, 2014, the FDA warned consumers not to buy or use weight loss supplement Bee Slim because it was found to contain sibutramine.
Recalls & Warnings
November 24, 2014
Weight Loss Supplement Contains Hidden Drug
On November 24, 2014 the FDA warned consumers not to buy or use weight loss supplement Super Extreme Accelerator because it was found to contain sibutramine.
Recalls & Warnings
November 21, 2014
Weight Loss Supplements Recalled
On November 19, 2014, REFA Enterprises, LLC issued a voluntary recall of one lot each of Forever Beautiful Bee Pollen and Forever Beautiful Infinity because they were found to contain sibutramine or a combination of sibutramine and phenolphthalein.
Recalls & Warnings
August 07, 2015
Weight Loss Supplement Found to Contain Drug
On August 6, 2015 the FDA warned consumers not to buy or use the weight loss supplement Achieving Zero because it was found to contain sibutramine.
Recalls & Warnings
April 08, 2015
Does Your Weight Loss or Sports Supplement Contain Synthetic Amphetamine?
A study published on April 7, 2015, reported that among 21 weight loss, sports and cognitive enhancement supplements labeled as containing the plant extract Acacia rigidula, more than half were found to contain a synthetic, amphetamine-like compound called beta- methylphenylethylamine ...
Recalls & Warnings
May 02, 2015
Drug Found in Two Weight Loss Supplements
The FDA recently warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine.
Recalls & Warnings
May 02, 2015
Three Sexual Enhancement Supplements Contain Prescription Drug
On April 30, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplements listed below because they were found to contain sildenafil. Each was identified during an examination of international mail shipments:
Recalls & Warnings
April 22, 2015
FDA Warns Consumers Not to Use Male Enhancement Supplement
On April 21, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Extreme Diamond 3000 because it was found to contain desmethyl carbodenafil and dapoxetine.
Recalls & Warnings
March 13, 2015
Weight Supplement Containing Drugs Recalled
On March 10, 2015, UltraZx, Labs, L.L.C, issued a voluntary recall of all lots of weight loss supplement UltraZx because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
March 11, 2015
Three Male Enhancement Supplements Found to Contain Drug
On March 3, 2015, the FDA warned consumers not to buy or use the following three sexual enhancement supplements because they were found to contain an undeclared sildenafil (each was identified during an examination of international mail shipments):
Recalls & Warnings
March 06, 2015
FDA Warns Consumers Not to Buy or Use Weight Loss Supplement Containing Hidden Drug
On March 5, 2015, the FDA warned consumers not to buy or use weight loss supplement L-Carnitine Sob Strengthening Version Slimming Miracle Capsule because it was found to contain sibutramine.
Recalls & Warnings
March 06, 2015
Four Male Enhancement Supplements Found to Contain Drug
On March 5, 2015, the FDA warned consumers not to buy or use the following four sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 06, 2015
Nine Male Enhancement Supplements Found to Contain Drugs
On March 2 and March 3, 2015, the FDA warned consumers not to buy or use the following nine male sexual enhancement supplements because they were found to contain an undeclared sildenafil or vardenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 03, 2015
Weight Supplement Contains Hidden Drug
On March 2, 2015, the FDA warned consumers not to buy or use weight loss supplement Elimulating Weight & Toxin Keeping Beauty because it was found to contain sibutramine.
Recalls & Warnings
March 02, 2015
Four More Sexual Enhancement Supplements Found To Contain Hidden Drug
On March 2, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 02, 2015
Hidden Drug Found in Six Sexual Enhancement Supplements
On February 27 and February 28, 2015, the FDA warned consumers not to buy or use the following six sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
September 26, 2015
Male Enhancement Supplement Containing Antidepressant Recalled
On September 25, 2015, TF Supplements issued a recall of two lots of the sexual enhancement supplement RHINO 7, which were found to contain the undeclared drugs desmethyl carbondenafil and dapoxetine.
Recalls & Warnings
October 24, 2015
Drug Found in Male Enhancement Supplement
On October 23, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement S.W.A.G.G.E.R Extreme because it was found to contain sildenafil.
Recalls & Warnings
October 17, 2015
"Herbal Viagra" Taken by Lamar Odom Was a Known Problem
It was reported this week that former NBA star, Lamar Odom, entered a coma after taking as many as 10 pills of the "herbal Viagra" formula Reload, along with cocaine.
Recalls & Warnings
October 14, 2015
Recall of Male Enhancement Supplement Expanded
On October 9, 2015, TF Supplements expanded its previous recall of two lots of RHINO 7 sexual enhancement supplements, which were found to contain the undeclared drugs desmethyl carbondenafil and dapoxetine, to include all lots.
Recalls & Warnings
October 02, 2015
Drug Found in Two Weight Loss Supplements
On October 1, 2015 the FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain sibutramine:
Recalls & Warnings
July 02, 2015
Undeclared Drugs Found in Two Weight Loss Supplements
On July 2, 2015 the FDA warned consumers not to buy or use weight loss supplements listed below because they were found to contain undeclared drugs. Click on the name of each product to read the full warning.
Recalls & Warnings
July 01, 2015
Undeclared Drugs Found In Male Enhancement Supplements
On June 10, 2015, the FDA issued a warning letter American Lifestyle, following tests which found the company's sexual enhancement supplements Vicerex and Sudibil-Xr to contain the undeclared drugs propoxyphenyl thioaildenafil and tadalafil.
Recalls & Warnings
June 06, 2015
Weight Supplement Recalled
On June 3, 2015 SmartLipo365 issued a voluntary recall of 122 lots of Smart Lipo because they were found to contain the undeclared drugs sibutramine, desmethylsibutramine, and phenolphthalein.
Recalls & Warnings
September 16, 2015
Weight And Enhancement Supplement Recall Expanded
On September 11, 2015, One Minute Miracle Inc. issued a recall of all lots the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
September 09, 2015
Weight Supplement Found to Contain Drug
On September 3, 2015 the FDA warned consumers not to buy or use the weight loss supplement Meizi Super Power Fruits Herbal Slimming Formula because it was found to contain sibutramine.
Recalls & Warnings
August 15, 2015
FDA Warns Seller of Tainted Enhancement Supplements
On July 31, 2015, the FDA issued a warning letter to R Thomas Marketing following a review of the company's website, which found it sold the male enhancement supplements Black Ant, Herb Viagra, Real Skill and Stree Overlord . These supplements contain the undeclared sildenafil.
Recalls & Warnings
June 22, 2017
FDA Finds Hidden Drug Ingredients in Sexual Enhancement Supplements
On June 22, 2017, the FDA warned consumers that the following sexual enhancement supplements were found to contain undeclared sildenafil, tadalafil and/or dapoxetine. Use the links below to read the complete warning letter for each:
- Triple Premium Zen Gold 1300 mg
Recalls & Warnings
May 26, 2017
"Allergy" Supplement Containing Ephedra Recalled
On February 7, 2017, MusclMasster, LLC (DBA The Green Herb) of Wheat Ridge, CO issued a recall of all bottles of Al-Er-G Capsules , a product promoted to help allergies, because they contain ephedra.
Recalls & Warnings
May 26, 2017
Herbal "Sexual Enhancement Coffee" Recalled, One Death Reported
On May 25, 2017, Caverflo.com issued a recall of Caverflo Natural Herbal Coffee, an "herbal" instant coffee promoted for sexual enhancement, because it was found to contain the drugs sildenafil and tadalafil.
Recalls & Warnings
April 19, 2017
Sexual Enhancement Supplements for Men and Women Recalled
On April 18, 2017, Organic Herbal Supply, Inc. issued a recall of all lots of the following sexual enhancement supplements for men because they were found to contain the undeclared drug tadalafil:
Recalls & Warnings
April 01, 2017
Weight Loss Supplement Containing Undeclared Drug Recalled
On March 28, 2017, Envy Me issued a recall of weight loss supplement LaBri's Body Health Atomic because it was found to contain undeclared sibutramine. Sibutramine, the active ingredient in the obesity drug Meridia, was removed from the U.S.
Recalls & Warnings
March 21, 2017
Twenty-one Sexual Enhancement Products Recalled
On March 7, 2017, A&H Focal Inc. issued a recall of the following sexual enhancement supplements because they were found to contain drugs such as sildenafil, tadalafil, vardenafil:
Recalls & Warnings
March 11, 2017
Two Hospitalized After Drinking Herbal Tea With Aconite
On March 11, 2017 the San Francisco Department of Public Health announced that two people became ill and required intensive hospital care after consuming tea made from tea leaves purchased from Sun Wing Wo Trading Company in San Francisco.
Recalls & Warnings
March 04, 2017
Sexual Enhancement Supplements Found to Contain Drugs
On March 3, 2017, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared drugs:
Recalls & Warnings
February 21, 2017
Sexual Enhancement Supplement Recalled
On February 16, 2017, Organic Herbal Supply, Inc. issued a recall of its sexual enhancement supplement XtraHRD Natural Male Enhancement because it was found to contain tadalafil.
Recalls & Warnings
February 09, 2017
Herbal Supplement Found to Contain Ephedra Alkaloids Recalled
On February 7, 2017, Kingsway Trading Inc. issued a recall of Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains ephedra alkaloids.
Recalls & Warnings
November 08, 2016
Weight Loss Supplements Contain Hidden Drugs, FDA Warns
The FDA warned consumers not to buy or use the weight loss supplements listed below because they were found to contain undeclared drugs:
Recalls & Warnings
December 03, 2016
Male Enhancement Supplement Recalled
On November 29, 2016, MS Bionic, Inc. issued a recall of all lots of Megajex Natural Male Sex Enhancer capsules because they were found to contain tadalafil and sildenafil.
Recalls & Warnings
February 04, 2017
FDA Warns Four Sexual Enhancement Supplements Contain Prescription Drug
The FDA recently warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared sildenafil.
Recalls & Warnings
February 04, 2017
Five Weight Loss Supplements Found to Contain Drugs
The FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs:
Recalls & Warnings
January 24, 2017
Weight Loss Supplements Found to Contain Drugs
The FDA warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs:
Recalls & Warnings
December 29, 2016
Sexual Enhancement Supplement Contains Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Power Male Sexual Stimulant because it was found to contain sildenafil. This supplement was identified during an examination of international mail shipments.
Recalls & Warnings
December 23, 2016
Twelve Sexual Enhancement Supplements Found to Contain Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the following twelve sexual enhancement supplements because they were found to contain undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
December 23, 2016
Weight Loss Supplement Found to Contain Antidepressant
On December 22, 2016, the FDA warned consumers not to buy or use the weight loss supplement Queen Slimming Soft Gel because it was found to contain undeclared fluoxetine and sibutramine.
Recalls & Warnings
November 11, 2016
Male Enhancement Supplement Contains Hidden Drug
On November 9, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Ready Man! because it was found to contain sildenafil and phenolphthalein.
Recalls & Warnings
November 11, 2016
FDA Warns Consumers Not to Use Weight Loss Supplement
On November 9, 2016, the FDA warned consumers not to buy or use the weight loss supplement Supreme Slim 5.7 because it was found to contain sildenafil and phenolphthalein.
Recalls & Warnings
August 20, 2016
Smart Lipo Weight Loss Supplement Found to Contain Undeclared Drugs
On June 16, 2016, the FDA issued a warning letter to Centro Naturista because the company's weight loss supplement, Smart Lipo, was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
July 03, 2016
Weight Loss Supplement Recalled
On June 1, 2016, Dream Body Weight Loss issued a recall of all lots of the following weight loss supplements listed because they were found to contain undeclared sibutramine:
- Dream Body Extreme Gold 800 mg 30 gold capsules
Recalls & Warnings
June 09, 2016
Nature Made Multis and B Complex Recalled Due to Salmonella, Staph Risk
On June 8, 2016 Pharmavite LLC issued a recall of the following Nature Made® multivitamin and B complex supplements because they have the potential to be contaminated with Salmonella or Staphylococcus aureus:
Recalls & Warnings
June 07, 2016
Step 2 Weight Loss Supplement Recalled
On June 7, 2016, The Body Shot Bar issued a recall of all lots of weight loss supplement Step 2 60 gold capsule (350 mg per) capsules which were distributed between March 1 through May 6 2016 because they were found to contain undeclared sibutramine.
Recalls & Warnings
May 15, 2016
Sexual Enhancement Supplements Recalled
On May 10, 2016, SOS Telecom, Inc. issued a recall of the following sexual enhancement supplements, which were found to contain undeclared sildenafil:
Recalls & Warnings
May 07, 2016
Weight Loss Supplement Contains Hidden Drug, FDA Warns
On May 6, 2016 the FDA warned consumers not to buy or use weight loss supplement Step 2 because it was found to contain undeclared sibutramine.
Recalls & Warnings
April 02, 2016
Propell Platinum Contains Undeclared Drugs
On March 29, 2016, the FDA warned consumers not to buy or use the weight loss supplement Propell Platinum because it was found to contain undeclared sibutramine and phenolphthalein.
Recalls & Warnings
March 29, 2016
Weight Loss Supplement Envy BP Contains Undeclared Drug
On March 28, 2016, the FDA warned consumers not to buy or use the weight loss supplement ENVY BP because it was found to contain undeclared sibutramine.
Recalls & Warnings
April 16, 2016
Workout Supplement Containing Acacia Rigidula Recalled
On March 18, 2016, Nubreed Nutrition, Inc. issued a recall of all lots of its "pre-workout" supplement Undisputed which is labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
April 13, 2016
"Super Herbs" Weight Loss Supplement Recalled
On April 11, 2016 Super Herbs issued a recall of all lots of weight loss supplement SUPER HERBS because it was found to contain sibutramine, desmethylsibutramine, and/or phenolphthalein.
Recalls & Warnings
March 20, 2016
Salute Capsules Contain Hidden Drugs
On March 17, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Salute Capsules because it was found to contain sildenafil, thiosildenafil, and sulfoaildenafil.
Recalls & Warnings
March 15, 2016
FDA Warns Sellers of Weight and Workout Supplements Containing Acacia Rigidula
On March 7, 2016, the FDA issued warning letters to five sellers of supplements which were labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
December 05, 2015
Lipo Escultura Weight Loss Capsules Recalled
On December 3, 2015 Lipo Escultura Corp. (doing business as JAT Productos Naturales Corp.) issued a voluntary recall of weight loss supplement Lipo Escultura capsules because they were found to contain sibutramine and diclofenac.
Recalls & Warnings
December 01, 2015
Enhancement Supplements Found to Contain Prescription Drug
On November 19, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements, which were identified during an examination of international mail shipments, because they were found to contain undeclared sildenafil (click on the name of each supplement to read the ...
Recalls & Warnings
November 10, 2015
"Natureal" Weight Supplement Recalled
On November 9, 2015 Inaffit, LLC issued a voluntary recall of all lots of weight loss supplement Natureal because it was found to contain undeclared sibutramine.
Recalls & Warnings
November 07, 2015
Power Khan Herbal Enhancement Supplement Found to Contain Drug
On November 5, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Power Khan because it was found to contain sildenafil.
Recalls & Warnings
November 28, 2015
FDA Warns Consumers Not to Buy or Use "Sex Drive Capsules"
On November 19, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Sex Drive Capsules because they were found to contain sildenafil.
Recalls & Warnings
November 21, 2015
Ultimate Herbal Slimcaps Recalled
On November 19, 2015 Fit Firm and Fabulous issued a voluntary recall certain lots of weight loss supplement Ultimate Herbal Slimcap capsules because they were found to contain sibutramine.
Recalls & Warnings
March 04, 2016
FDA Warns Consumers Not To Use Sexual Enhancement Supplement
On March 3, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Sextra because it was found to contain sildenafil.
Recalls & Warnings
February 27, 2016
Enhancement Supplement Contains Undeclared Drug
The FDA recently warned consumers not to buy or use the sexual enhancement supplement Neophase Natural Sex Enhancer because it was found to contain hydroxyacetildenafil.
Recalls & Warnings
January 31, 2016
Pink Bikini and Shorts on the Beach Weight Supplements Recalled
On January 28, 2015, Lucy's Weight Loss System issued a voluntary recall of all lots of weight loss supplements Pink Bikini (white capsules, blue capsules and gold capsules) and Shorts on the Beach (blue capsules and gold capsules) because they contain undeclared sibutramine, ...
Recalls & Warnings
January 22, 2016
Male Enhancement Gum and Pills Contain Hidden Drug
On January 21, 2016, the FDA warned consumers not to buy or use the following sexual enhancement supplements, because they were found to contain undeclared vardenafil (click on the name of each supplement to read the FDA's complete warning):
Recalls & Warnings
January 12, 2016
Twenty-four Male Enhancement Supplements Recalled
On January 9, 2016, R Thomas Marketing LLC. in conjuction with Just Enhance LLC, issued a recall of the following sexual enhancement supplements because they were found to contain sildenafil:
Recalls & Warnings
December 23, 2015
"Bee Extremely Amazed" Recalls Weight Supplements
On December 22, 2015, Bee Extremely Amazed LLC issued a voluntary recall of all lots of the following weight loss supplements, which were found to contain sibutramine and phenolphthalein:
Recalls & Warnings
December 19, 2015
"Tiger X" and "X Again Platinum" Contain Hidden Drugs
On December 17 and 18, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements, which were found to contain undeclared drugs:
Recalls & Warnings
December 19, 2015
Don't Get Stung by Bee Pollen
On December 18, 2015, the FDA warned consumers not to buy or use the following supplments promoted for weight loss because they were found to contain sibutramine and phenolphthalein:
Recalls & Warnings
December 19, 2015
"Thirty Plus" Contains Hidden Drug
On December 18, 2015, the FDA warned consumers not to buy or use the weight loss supplement Thirty Plus because it was found to contain sibutramine.
Recalls & Warnings
December 19, 2015
Drugs Found in Jenesis Weight Supplement
On December 18, 2015, the FDA warned consumers not to buy or use the weight loss supplement Jenesis because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
September 26, 2014
Sellers of Essential Oils Warned for Claiming to Treat Ebola, Other Diseases
On September 22, 2014, the FDA issued a warning to Young Living following a review of websites, Pinterest, Facebook and Twitter accounts owned by Young Living distributors, which found statements made about the company's essential oils, including Thieves, Cinnamon Bark, Oregano, ImmuPower, ...
Recalls & Warnings
November 02, 2012
Manufacturer of Prostate, Inflammation and Cholesterol Supplements Warned For Drug Claims
On October 15, 2012, the FDA issued a warning letter to Ortho Molecular Products, Inc. for making drug claims about the following dietary supplements: Candicid Forte, Inflamma-bLOX, Lipitrol, Mucosagen, Prostatrol Forte, Resvoxitrol, Paracid Forte, VascuPak HTN and Lithium Orotate.
Recalls & Warnings
February 22, 2018
Kratom Supplements Recalled and Destroyed
On February 21, 2018, the FDA announced the voluntary destruction and recall of Botany Bay, Enhance Your Life and Divinity kratom supplements. All of the products were manufactured and distributed nationwide by Divinity Products Distribution, located in Grain Valley, Missouri.
Recalls & Warnings
February 20, 2018
Salmonella Outbreak Linked to Kratom Powders, Pills and Teas
On February 20, 2018, the Center for Disease Control (CDC) warned consumers not to use kratom in any form because it could be contaminated with Salmonella. The CDC is currently investigating an outbreak of illness caused by Salmonella infection associated with the use of kratom.
Recalls & Warnings
January 26, 2018
Seller of Coenzyme-A Warned For Making Drug Claims
On January 5, 2018, the FDA issued a warning letter to Coenzyme A, Inc. following a facility inspection and review of the company's website that found the company made drug claims about its product Coenzyme-A.
Recalls & Warnings
July 01, 2005
FDA Issues Nationwide Alert for "Liqiang 4" Due to Potential Health Risk
On July 1, 2005, the U.S. Food and Drug Administration (FDA) warned consumers not to take Liqiang 4 Dietary Supplement Capsules because they contain glyburide – a drug that could have serious, life-threatening consequences in some people.
Recalls & Warnings
February 10, 2018
Warning to D-Limonene, Vitamin C Seller
On January 31, 2018 the FDA issued a warning letter to Long Life Unlimited, LLC following a review of the company's website which found promotional statements and testimonials made about products including Balance 600, D-Limonene, Rapha Remedy, Rapha Remedy w/ p73 Wild Oregano, Vitamin C, ...





