Reviews and Information for Global Pharmaceuticals
Search term may appear only in full report available to members. Join now for full access.
Product Review
Wellbutrin vs. Generic Bupropion Review
Generic Bupropion Is Not Always The Same As Name-Brand Wellbutrin. CL Tests Reveal Differences Between Generic Bupropion and Name-Brand Wellbutrin.
Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

Recalls & Warnings
February 06, 2023
Recalled Eye Drops Linked to Vision Loss and One Death
On February 2, 2023, Global Pharma Healthcare issued a nationwide recall of its Artificial Tears Lubricant Eye Drops due to potential contamination with bacteria that can cause serious infection, blindness, and death.
Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Pumpkin Seeds (Pepitas) Review
We Identified the Best Pumpkin Seeds

Product Review
Sunflower Seeds and Butters Review
High Levels of Toxin Found in Most Sunflower Seeds and Butters

Product Review
Ashwagandha Supplements Review
Find the Best Ashwagandha Supplement. Only 38% of Ashwagandha Products Pass Tests.

Product Review
Aloe Juices, Gels, and Supplements Review
How Much Aloe is Really in Aloe Products? Find Out and See Our Top Picks.

CL Answer
Which supplements have been shown to be helpful for autism?
Find out which supplements may help reduce symptoms of autism, including multivitamins, probiotics, and melatonin.

Recalls & Warnings
December 14, 2023
Pain Relief Tea Recalled, Found to Contain Prescription Drugs
On December 13, 2023, WS Global issued a recall of all lots of the company’s Himalayan Pain Relief Tea after products were found to contain diclofenac and dexamethasone, prescription drugs that are not listed on the product’s label.
Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

Clinical Update
3/31/2023
Olive Oil Review Expanded: Many Do Not Make the Grade
We tested three new olive oils based on reader requests, and the results are in! Our new tests included two olive oils that claim to be high in polyphenols, and the results were surprising:
-- One olive oil was rated so poorly by our professional taster it was considered to be "lampante" -- i.e., not fit for human consumption.
-- The other high-polyphenol oil delivered a good amount of polyphenols, rated well for taste, and is now our new Top Pick for a very high-polyphenol olive oil.
We also tested California Olive Ranch Global Blend but were somewhat disappointed.
See the results now for 13 popular olive oils in our updated Extra Virgin Olive Oil Review.
Recalls & Warnings
December 01, 2021
Probiotics Recalled Due to Bacterial Contamination, May Be Life-Threatening to Some
On December 1, 2021, Livia Global, Inc issued a voluntary recall of two lots of probiotics due to potential contamination with Pseudomonas aeruginosa. This bacterium, if ingested, can cause life-threatening infection in immunocompromised individuals.
Recalls & Warnings
January 04, 2024
Toxic Herb Found in More Tejocote Root Supplements
On January 3, 2024, the FDA warned consumers not to purchase or use certain tejocote root supplements after FDA laboratory analysis confirmed the products contain yellow oleander (Thevetia peruviana), a toxic herb.
CL Answer
What is hesperidin, what is it used for and is it safe?
Learn about hesperidin, including whether its beneficial for heart health, problems with circulation, stroke, cognitive function, depression, cancer and other conditions, and find out if it is safe.

Recalls & Warnings
March 13, 2020
FDA Finds Problems at 52% of Supplement Manufacturing Sites in U.S. and 42% Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2019 (October 1, 2018 - September 30, 2019) of 598 dietary supplement manufacturing facilities in the U.S.
CL Answer
Can I get the new coronavirus (2019-nCoV) from supplements from China?
Information about catching the coronavirus from supplements from China, plus info on contamination with heavy metals like lead and more.

CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

News Release
April 10, 2023
Best and Worst Olive Oils According to ConsumerLab Tests
White Plains, New York, April 10, 2023 — Extra virgin olive oil can be a heart-healthy alternative to saturated fats in the diet, providing antioxidant polyphenols and plenty of oleic acid, a “healthy” monounsaturated fat.
News Release
April 16, 2008
FDA's investigation of generic antidepresssant called inadequate by ConsumerLab.com and The People's Pharmacy
WHITE PLAINS, NEW YORK AND DURHAM, NORTH CAROLINA — APRIL 16, 2008 — After waiting more than six months for results of the FDA's investigation of a generic version of Wellbutrin XL 300 (Budeprion XL 300), ConsumerLab.
News Release
March 17, 2008
As problems surface, Consumerlab.com and the people's pharmacy ask FDA to disclose differences between generic and original drugs
WHITE PLAINS, NEW YORK AND DURHAM, NORTH CAROLINA — MARCH 17, 2008 — An investigation by ConsumerLab.
CL Answer
L-Serine & D-Serine: Health Benefits and Safety
Serine exists in two forms: L-serine and D-serine. Find out how these two forms differ and learn if either form has health benefits and if it is safe.
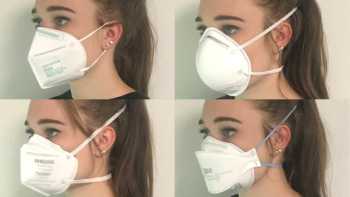
CL Answer
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

Recalls & Warnings
March 02, 2021
FDA Warns Seller of Vitamin C, Silver Spray
On March 1, 2021, the FDA issued a warning letter to Ageless Global, LLC following a review of the company's websites for selling Immunoral, Immune Plus, MD Immune Support Spray, and MD CVK-365 Mouth Spray with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
February 02, 2021
Seller of Aloe Products Warned for Claiming to Treat Joint Stiffness
On January 22, 2021, the FDA issued a warning letter to American Global Health Group, LLC following a review of the company's website, which found statements made about the company's products AloeCure VeraFlex, AloeCure Advanced Formula Capsule, AloeCure Pure Aloe Vera ...
Recalls & Warnings
May 08, 2024
Razer, Inc. to Pay Over $1.1 Million Settlement for Zephyr Face Mask ”N-95” Claims
On April 29, 2024, the FTC announced it will be returning over $1.1 million to consumers who purchased Zephyr face masks after the company promoted its Zephyr face masks as N95-grade despite never submitting for testing to the FDA for approval of such claim.
News Release
October 12, 2007
ConsumerLab.com finds generic antidepressant behaves differently from original drug. May explain complaints by patients. — Generic drug equality questioned
WHITE PLAINS, NEW YORK AND DURHAM, NORTH CARLOLINA — OCTOBER 12, 2007 — ConsumerLab.
News Release
August 15, 2001
Problems and ambiguity among alternative estrogen products reported by ConsumerLab.com — Results of soy and red clover isoflavone product testing released online today
WHITE PLAINS, NY — August 15, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Phytoestrogen Product Review. The review focused on dietary supplements made from soy and red clover isoflavones.
Recalls & Warnings
July 30, 2014
Chocolate and Carob Drink Mix Recalled Due To Salmonella Risk
On July 25, 2014, CaCoCo, Inc. issued a recall of CaCoCo Original and Global Warrior chocolate drink mixes because they contain organic carob powder which has the potential to be contaminated with Salmonella.
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
Recalls & Warnings
July 19, 2011
Recall of Enhancement Supplement for Men - Contains Drug Compounds
On July 13, 2011, Global Wellness, LLC announced an expanded voluntary nationwide recall of Via Extreme Ultimate Sexual Enhancer Dietary Supplement for Men. The product was distributed throughout the U.S. Puerto Rico, Barbados, and Canada to internet and retail consumers.
Recalls & Warnings
August 18, 2020
Seller of Immune Shot Criminally Charged With Making Coronavirus Claims
On August 10, 2020, prosecutors in Georgia charged Matthew Ryncarz and his company Fusion Health and Vitality, LLC d/b/a/ Pharm Origins with selling the misbranded product Immune Shot.
Recalls & Warnings
May 02, 2020
Seller of Botanical and CBD Oil Patches Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Santiste Labs LLC for promoting its transdermal patches containing botanical oils and/or CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
January 02, 2012
Manufacturing Violations for Multivitamin, Vitamin K and Other Supplements
On December 19, 2011, the U.S. FDA sent a Warning Letter to Milk Specialties Global regarding manufacturing violations. Affected products include PrimaForce Proliver Capsules, Dr. Mercola Vitamin K2, Active Women’s Multivitamin/Multimineral, Cremagnavol, Immune Support, and HPF Women’s Multi.
Recalls & Warnings
March 04, 2003
U.S. Marshalls Seize Supplements Claiming to Prevent Cancer and Treat Arthritis
On February 12, 2003, at the request of the Food and Drug Administration (FDA), U.S. Marshals seized dietary supplement products from Global Source Management and Consulting, Inc., in Sunrise, Florida. U.S.
Recalls & Warnings
April 27, 2016
Multivitamin Recalled Due to Undeclared Allergens
On April 19, 2016, Nuvi Global Corporation issued a recall of StemVitae 30oz liquid multivitamin because they contain the undeclared milk and soy lecithin.
Recalls & Warnings
April 23, 2019
"Brain Boosting" Supplements Were Promoted With Non-Existent Clinical Studies
On April 10th, 2019, the FTC (Federal Trade Commission) announced the marketers of cognitive enhancement supplements Geniux, Xcel, EVO, and Ion-Z have agreed to settle charges that they made false claims about the product, including fake research references and celebrity ...
Recalls & Warnings
May 01, 2014
Weight Loss Supplement Recalled
On April 29, 2014, dietary supplement distributor Bacai Inc. issued a voluntary recall of one lot of weight loss supplement LiteFit USA because it was found to contain sibutramine.
Recalls & Warnings
January 18, 2013
FTC Upholds Ruling, POM Wonderful Health Claims Were Deceptive
On January 16, 2013, the Federal Trade Commission (FTC) announced it will uphold a judge’s ruling that makers of POM Wonderful 100% Pomegranate Juice and POMx supplements made deceptive and unsubstantiated claims that the products could treat, prevent, or reduce the risk of heart disease, prostate ...
Recalls & Warnings
March 02, 2016
Seller of Hoodia and Other Supplements Admits Fraud
On March 1, 2016, The United States Department of Justice announced that David Romeo, principal of several New Jersey- based dietary supplement companies, including Global Nutrients, Stella Labs and Nutraceuticals International, LLC, has pled guilty to conspiracy to distribute three kilograms or ...
Recalls & Warnings
March 13, 2015
Problems Found with New Zealand Fish Oil Supplements, Concerns Raised About Testing Methods
On January 21, 2015, a study was published which reported that only 3 of 32 fish oil supplements commercially available in New Zealand contained the amount of EPA and DHA listed on their labels (Albert, Sci Rep 2015).
Recalls & Warnings
July 14, 2020
Forty-Six More Hand Sanitizers May Contain Toxic Ingredient, Ten More Recalled
Between July 6 and 10, 2020, the FDA announced that forty-six more hand sanitizers may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
February 27, 2009
$4 Million Settlement by Supplement Maker for False Claims
On February 26, 2009, the Texas Attorney General announced that an agreement was reached with Mannatech Inc. and its former CEO, Samuel L. Caster, both of which had been charged with orchestrating an unlawful marketing scheme that exaggerated their products’ health benefits.
Recalls & Warnings
April 29, 2012
FDA Says DMAA Can't Be Sold as a Supplement -- Warns Sellers
On April 27, 2012, the U.S.
Recalls & Warnings
March 12, 2016
FDA Finds Problems at 58% of Supplement Manufacturing Sites in U.S. and Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2015 (ending September 30) of 483 dietary supplement manufacturing facilities, showing that most -- 58.2% -- received letters indicating noncompliance with current Good Manufacturing Practices (cGMPs).





