Reviews and Information for Ginkgo Biloba
Ginkgo Supplements
Learn how to find the best Ginkgo biloba supplements, amounts of flavonol glycosides and terpene lactones found in popular products and testing for contamination. Read our review to find out if Ginkgo helps for memory, dementia, Alzheimer's disease, vertigo, glaucoma and other conditions. Review information about dosage, safety, side effects and more. Find out which ones passed our tests and why.
Ginkgo Supplements ReviewSearch term may appear only in full report available to members. Join now for full access.
Product Review
Sexual Enhancer Supplements Review (with Yohimbe, Horny Goat Weed, Arginine)
Choose the Best Sexual Enhancer Supplement. Only 30% of Selected Sexual Enhancement Supplements Pass Quality Tests.

News Release
March 07, 2018
Finding Real Ginkgo Isn't Easy, ConsumerLab's Tests Reveal
White Plains, New York, March 7, 2018 — Recent tests of popular Ginkgo biloba supplements sold in the U.S. found that only 4 of 10 were confirmed to contain their listed amounts of real ginkgo extract.
Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

Clinical Update
4/21/2013
Does Ginkgo Cause Cancer?
According to a new study from the government, animals given an extract of Ginkgo biloba for two years developed cancers of the thyroid and liver. Should you be concerned? See the "Concerns and Cautions" section of the Ginkgo Biloba Supplements Review for more information, as well our assessment of ginkgo's usefulness, and our tests of ginkgo supplements
Clinical Update
9/09/2012
Ginkgo Fails Alzheimer's Trial
Over two thousand people with memory complaints were given Ginkgo biloba extract or placebo as part of a five-year study. The results, published this week, showed no difference between the groups in the percentage who eventually developed Alzheimer's disease or dementia. See how this compares to other studies of ginkgo in the updated Ginkgo Biloba Supplements Review, which includes our tests and reviews of ginkgo products. More >>
Clinical Update
8/17/2016
Ginkgo for Memory and Cognition?
A branded ginkgo biloba extract improved a measure of cognition in men and women with self-reported memory impairment -- but did not improve memory. Get the details, plus more about the evidence for ginkgo and our tests of products, in the Ginkgo Biloba Supplements Review >>
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
CL Answer
Supplements for multiple sclerosis (MS): Do any supplements reduce symptoms or relapse?
Find out if vitamins or supplements such as ginkgo, fish oil, biotin, vitamins A, C, E, D, and B12, or selenium reduce symptoms or relapse in people with multiple sclerosis (MS).
CL Answer
I have been having dizziness for the past few months and am wondering if it could be a side effect of supplements I take. Which supplements cause dizziness?
Find out which supplements that may cause dizziness, vertigo, or imbalance, including garlic, melatonin, saw palmetto, and red yeast rice.

CL Answer
Are there supplements, foods, or beverages that I should avoid when taking clopidogrel (Plavix)?
Learn about supplement interactions with clopidogrel (Plavix). Supplements that may interact with this medication include cannabidiol (CBD), grapefruit, St. John's wort, ginkgo and others.

CL Answer
Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Find out which supplements help improve memory, brain function and cognition, including fish oil, some B vitamins, cocoa, and curcumin. ConsumerLab's answer explains the evidence for supplements promoted to help with brain function and cognition.
CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

CL Answer
Have any supplements been shown to increase women's libido?
Learn more about supplements that may increase women's libido, including DHEA and maca.

CL Answer
Are supplements which claim increased absorption or improved bioavailability telling the truth? Is it worth paying more for these? Are there concerns?
Learn more about bioavailability enhancers, including emulsifiers and enzyme inhibitors.

Clinical Update
9/30/2012
Does Ginkgo Improve Memory?
The evidence for Ginkgo biloba in preventing and treating Alzheimer's disease and other dementias has become fairly negative. But is there still hope for its use in improving memory in healthy individuals? The answer seems to be in, according to a new analysis of 10 placebo-controlled trials in healthy people. Get the answer in the update to the Ginkgo Supplements Review.
Clinical Update
11/16/2019
Concern With Ginkgo
The use of Ginkgo biloba has been associated with an electrolyte imbalance. For details, see the Concerns and Cautions section of the Ginkgo Supplements Review. Also see our Top Pick for Ginkgo.
Clinical Update
12/12/2017
Skin Rash from Ginkgo
Although not common, be aware that skin reactions have been reported from taking Ginkgo biloba. The most recent report involved a young man who experienced symptoms the same day he began taking a popular ginkgo supplement. See the details in the "Concerns and Cautions" section of the Ginkgo Supplements Review >>
CL Answer
Do any supplements help reduce menstrual pain or symptoms of premenstrual syndrome (PMS)?
Find out which supplements help reduce premenstrual syndrome (PMS) symptoms, such as mood swings, pain, bloating, irritability and food cravings.

CL Answer
Which supplements help with Raynaud's phenomenon?
Raynaud's phenomenon may be helped by some supplements. Learn more about fish oil, Gingko biloba, and ginseng.

Clinical Update
8/26/2024
Supplement for Memory?
Did Nutrilite Memory Builder improve memory and cognition in healthy, older men and women in a recent study? Find out in the Memory section of our Gingko Biloba Supplements Review, which includes our Top Pick for gingko.
Also see: Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
CL Answer
Which supplements reduce the risk of stroke? Which increase the risk of stroke?
Find out which supplements (may help reduce the risk of stroke/might increase the risk of stroke.)

Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

News Release
November 18, 2008
Adulteration Suspected with Some "Memory" Supplements -- Few Ginkgo and Huperzine Supplements Pass ConsumerLab.com Tests; Quality High for Acetyl-L-Carnitine
WHITE PLAINS, NEW YORK — NOVEMBER 18, 2008 — Tests by ConsumerLab.com of Ginkgo biloba supplements show that few products meet quality standards.
CL Answer
Are there any supplements I should avoid when taking acetaminophen (Tylenol)?
Find out if there are any supplements that should be avoided when taking acetaminophen (Tylenol).
CL Answer
What supplements should I stop taking before surgery?
Info on which supplements to avoid before surgery due to increased risk of bleeding or interference with anesthesia.
CL Answer
Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Find out which supplements for nerve pain, including fish oil and alpha-lipoic acid, are effective for sciatica, diabetic neuropathy, or postherpetic neuralgia.

Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

CL Answer
Dietary Supplements and Herbs to Avoid When Breastfeeding
Find out which supplements should be avoided when breastfeeding, including medicinal herbs, soy, and flaxseed.

News Release
May 24, 2006
Problems persist with ginseng supplements — ConsumerLab.com finds nearly half of products lack ingredient or contaminated
WHITE PLAINS, NEW YORK — May 24, 2006 — ConsumerLab.com has released test results for dietary supplements made with ginseng, a popular herb promoted for vitality and the treatment or prevention of a range of medical conditions.
Product Review
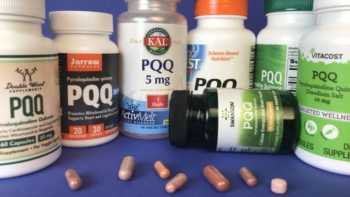
PQQ (Pyrroloquinoline Quinone) Supplements Review
Learn What PQQ Does and Which PQQ Supplements are Best
CL Answer
What are "broad-spectrum micronutrient" supplements, and do they have greater health benefits than multivitamin/multimineral supplements?
Learn about "broad-spectrum micronutrients" and how they compare to multivitamin/multimineral supplements.
CL Answer
Which supplements help with erectile dysfunction?
Supplements for erectile dysfunction. Find out which supplements can help with ED, including ginseng, L-arginine, and maca.

News Release
January 03, 2006
Tests of memory enhancing supplements by ConsumerLab.com reveals lead in some ginkgo
WESTCHESTER COUNTY, NEW YORK — TUESDAY, JANUARY 3, 2006 — In its new test report on Memory Enhancement Supplements, ConsumerLab.com has revealed finding significant amounts of lead in certain products on the market.
Product Review
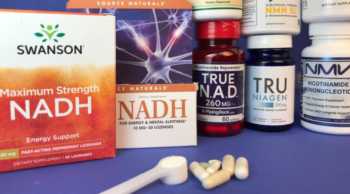
NAD Booster Supplements Review (NAD+/NADH, Nicotinamide Riboside, and NMN)
How Important Is Boosting NAD+ Levels? Find Out and Learn How Booster Supplements Compare.
Product Review
Choline and Lecithin Supplements Review (Including Phosphatidylcholine, CDP-Choline, and Alpha-GPC)
Choose the Best Choline Supplement. Find Out How Much Choline Popular Supplements Really Provide.

Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

Recalls & Warnings
December 31, 2005
Ginkgo Supplements Recalled
On December 28, 2005, Jarrow Formulas issued a recall of a line of Ginkgo biloba supplements because raw material potency is below the label claim. Affected are "Ginkgo biloba 50:1" products sold in 40, 60, and 120 mg strengths and a combination product "Ginkgo Biloba 50:1 + Grape OPCs, 60 mg."
CL Answer
Does Qualia Mind boost memory and mental function and is it worth cost?
Learn about the ingredients in Qualia Mind, whether or not we think the formula works and is safe, and if we think it's worth the cost.

News Release
November 15, 2005
Testing by ConsumerLab.com identifies many problems with popular supplements for weight loss, slimming, and blood sugar control
Westchester, NY — November 15, 2005 — In a series of reports released today, ConsumerLab.com revealed test results for supplements used for weight loss, slimming, and blood sugar control. Among the twenty-three products that ConsumerLab.
News Release
August 31, 2005
ConsumerLab.com testing of alpha lipoic acid supplements from Japan finds no ingredient in one product; Most others meet claims — Japanese consumers urged to research product quality
WHITE PLAINS, NY — ConsumerLab.com reported this week that among nine brands of alpha lipoic acid supplements it recently purchased in Japan and tested, one contained none of the popular ingredient and another contained only 81%.
News Release
August 16, 2005
ConsumerLab.com tests potassium supplements — Report now online for sixteen products
WHITE PLAINS, NY — August 16, 2005 — ConsumerLab.com has released a new report on the quality of potassium supplements. Potassium is used to treat or prevent potassium deficiency caused by diuretic drugs ("water pills"), prolonged vomiting, diarrhea or laxative abuse.
Recalls & Warnings
November 16, 2018
Two Popular Memory Supplements Missing Some or All of Key Ingredient
Two popular Ginkgo biloba supplements marketed to improve memory do not contain the Ginkgo biloba claimed on their labels, according to tests conducted by the U.S. Government Accountability Office (GAO).
News Release
July 18, 2005
ConsumerLab.com tests menopause supplements — Alternatives to hormonal therapy — Report available for 38 products including soy and red clover isoflavones, black cohosh, and progesterone creams
WHITE PLAINS, NY — July 18, 2005 — ConsumerLab.com has released a new report on the quality of supplements used to treat symptoms of menopause, notably hot flashes.
News Release
July 12, 2005
ConsumerLab.com finds some supplements lower in omega-3 and omega-6 fatty acids than claimed — Report available for 23 products made from oils of black currant, borage, evening primrose and flax
WHITE PLAINS, NY — July 12, 2005 — ConsumerLab.com has released its latest report on the quality of supplements made from the seed oils of black currant, borage, evening primrose, and flax.
News Release
June 20, 2005
Quality problems widespread among ginseng products sold in Japan. — Nearly two-thirds of products fail ConsumerLab.com testing due to pesticide contamination and missing or substandard ingredients
WHITE PLAINS, NY — JUNE 20, 2005 — Among 14 brands of ginseng dietary supplements recently purchased in Japan and tested by ConsumerLab.com, only 5 products passed independent testing by ConsumerLab.com.
News Release
June 15, 2005
Two magnesium supplements fail testing due to lead contamination. Nineteen others pass. Report now available.
WHITE PLAINS, NY — JUNE 15, 2005 — ConsumerLab.com has released its latest report on the quality of magnesium supplements sold in the U.S. In addition to being an essential mineral, magnesium may help prevent migraine headaches, menstrual pain, PMS, and leg cramps during pregnancy.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
CL Answer
Does Prevagen really improve memory?
Learn more about Prevagen, including clinical studies on Prevagen's impact on memory & cognition, safety, pricing, and an FDA warning letter. ConsumerLab explores the efficacy of Prevagen.

News Release
May 10, 2005
ConsumerLab.com begins publishing test reports on Japanese vitamins and supplements — Review of CoQ10 products Now Available Online in Japanese at ConsumerLab.jp. More Reports Scheduled
— Review of CoQ10 Products Now Available Online in Japanese at ConsumerLab.jp. More Reports Scheduled — WHITE PLAINS, NY — MAY 10, 2005 — ConsumerLab.
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
News Release
December 14, 2004
ConsumerLab.com expands testing to Japan — Testing Organization Expands to Help Japanese Consumers Determine Quality of Health and Nutrition Products
WHITE PLAINS, NY — Tuesday, December 14, 2004 — ConsumerLab.com announced today that it would begin testing products sold in Japan. Its first report is due out in early spring. In the U.S. and Canada, ConsumerLab.
News Release
August 28, 2003
Some problems persist with ginseng supplements but overall quality improves according to ConsumerLab.com — Test results published online today
WHITE PLAINS, NY — August 28, 2003 — In contrast to its testing three years ago in which nearly 60% of Asian and American Ginseng supplements were found to have significant problems, testing of recently purchased products by ConsumerLab.
News Release
April 21, 2003
Low quality ingredient appears widespread among Ginkgo supplements according to ConsumerLab.com; points to challenge for FDA's proposed regulations — Review of memory enhancement supplements released today
WHITE PLAINS, NY — April 21, 2003 — In contrast to its findings three years ago, ConsumerLab.com announced today that only 22% of the Ginkgo biloba supplements it recently tested met its quality standards. In late 1999, 75% of the products it tested met these standards.
News Release
January 21, 2003
Acidophilus" and other probiotic supplements gain in popularity but live bacteria missing in many — ConsumerLab.com releases review online today
WHITE PLAINS, NY — January 21, 2003 — ConsumerLab.
News Release
December 30, 2002
Top quality melatonin products identified by ConsumerLab.com — Hormone supplement used to treat sleep disturbances due to jet travel and other causes
WHITE PLAINS, NY — December 30, 2002 — In its latest Product Review, ConsumerLab.com found that 16 of 18 melatonin dietary supplements met their label claims and were free of lead contamination.
News Release
December 10, 2002
Lupus patients cautioned about hormone supplement — ConsumerLab.com releases DHEA testing results online today
WHITE PLAINS, NY — December 10, 2002 — In its latest Product Review, ConsumerLab.com found that 3 of the 17 DHEA (dehydroepiandrosterone) supplement products it tested contained less than their claimed amounts of this hormone — one having less than one-fifth of what it claimed.
News Release
July 10, 2002
Pharmavite dietary supplements receive ConsumerLab.com approval — Vitamin E, SAM-e, St. John's Wort, Ginkgo and others merit approved quality products ranking
WHITE PLAINS, NY — July 10, 2002 — ConsumerLab.
News Release
February 08, 2002
Athletic Banned Substances Screening and Certification Program announced by ConsumerLab.com — Tester of supplements to screen for substances banned in Olympics
WHITE PLAINS, NY — February 8, 2002 — ConsumerLab.
News Release
January 08, 2002
ConsumerLab.com finds most B vitamin supplements contain what they claim, but often exceed safe levels — Consumers cautioned to be aware of side effects with high dose products
WHITE PLAINS, NY — January 8, 2002 — ConsumerLab.com announced today that all but one of the twenty-one products analyzed in its B Vitamin Product Review contained the claimed amounts of B vitamins.
News Release
November 20, 2001
ConsumerLab.com finds fish oil supplements free of mercury, but 30% lacking in key ingredient — Test results of omega-3 fatty acid (EPA and DHA) products released today
WHITE PLAINS, NY — November 20, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Product Review of Omega-3 Fatty Acids (EPA & DHA) from Fish/Marine Oils.
News Release
October 30, 2001
Sixty percent of nutrition bars fail to meet claims in ConsumerLab.com tests — "Low Carb" bars often loaded with carbohydrates; excess sodium and saturated fat also found
WHITE PLAINS, NY — October 30, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Nutrition Bar Product Review.
News Release
October 03, 2001
Most iron supplements pass ConsumerLab.com testing — Lead contamination and insufficient iron found in some
WHITE PLAINS, NY — October 3, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Product Review of Iron Supplements. Iron deficiency is the most common cause of anemia in the U.S. and worldwide.
News Release
August 15, 2001
Problems and ambiguity among alternative estrogen products reported by ConsumerLab.com — Results of soy and red clover isoflavone product testing released online today
WHITE PLAINS, NY — August 15, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Phytoestrogen Product Review. The review focused on dietary supplements made from soy and red clover isoflavones.
News Release
July 09, 2001
Many herbal sleep products lack key claimed ingredient — Results of ConsumerLab.com's testing of valerian products released on web today
WHITE PLAINS, NY — July 9, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of valerian supplements — used primarily as sleep aids and minor tranquilizers.
Recalls & Warnings
September 10, 2012
Maker of Concussion Recovery Supplement Warned For Making Drug Claims
On August 28, 2012, the FDA issued a warning letter to Trinity Sports Group, Inc. after a review of the company's websites found statements about Neuro Impact Concussion Response Formula constituted drug claims not permitted on supplements.
News Release
June 04, 2001
ConsumerLab.com reports test results of arthritis supplement — MSM; Quality found higher than for most supplements, but room for improvement remains
WHITE PLAINS, NY — June 4, 2001 — ConsumerLab.
News Release
May 07, 2001
Over forty percent of Echinacea products fail ConsumerLab.com review; Inadequate labeling and missing ingredients found common for popular herbal cold remedy
WHITE PLAINS, NY — May 7, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of echinacea supplements.
News Release
April 10, 2001
Quality of popular herbal anti-depressant found to vary; certain types of St. John's Wort supplements more likely to pass testing
White Plains, NY — April 11, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of St. John's wort supplements.
News Release
March 27, 2001
ConsumerLab.com expands testing of health products; success of dietary supplement evaluations leads company into broader nutrition field, beginning with popular nutrition bars
WHITE PLAINS, NY — March 27, 2001 — Having established its presence as the leading independent evaluator of the quality of dietary supplements sold in the U.S, ConsumerLab.com announced today the expansion of its work to include foods and food ingredients.
News Release
March 13, 2001
ConsumerLab.com review of vitamin E supplements finds some insufficiencies and need for clearer labeling; test results released online today
WHITE PLAINS, NY — March 13, 2001 — ConsumerLab.com today released results of its Product Review of vitamin E products. Vitamin E is one of the most popular dietary supplements in the U.S.
News Release
February 14, 2001
Problems found with multivitamins and multiminerals by ConsumerLab.com
WHITE PLAINS, NY — February 14, 2001 — ConsumerLab.com announced today that only 14 of 27 products evaluated in its Multivitamin and Multimineral Product Review achieved full "CL Approved Quality" status.
News Release
January 29, 2001
Some supplements for arthritis may exceed newly released safe intake levels for manganese
WHITE PLAINS, NY — January 29, 2001 — ConsumerLab.com issued an alert today to its online readers that that some glucosamine and chondroitin products (popularly used for osteoarthritis), exceed newly established upper intake levels for manganese.
News Release
November 21, 2000
ConsumerLab.com tests popular supplement for heart failure; CoQ10 test results released online today
WHITE PLAINS, NY — November 21, 2000 — ConsumerLab.com today released results of its 10th Product Review, focusing on dietary supplements containing coenzyme Q10 (CoQ10).
News Release
September 06, 2000
ConsumerLab.com finds new calcium products of higher quality than many traditional supplements; Test results released online today
WHITE PLAINS, NY, September 6, 2000 — ConsumerLab.com today released results of its 9th Product Review, focusing on dietary supplements and products providing calcium. One of the most commonly purchased supplements in the U.S.
News Release
August 07, 2000
ConsumerLab.com finds that not all creatine supplements meet label claims; Popular sports supplement test results released online
WHITE PLAINS, NY, August 7, 2000 — ConsumerLab.com today released results of its 8th Product Review, focusing on creatine monohydrate dietary supplements.
News Release
July 11, 2000
Pesticide contamination found in many Ginseng supplements tested by ConsumerLab.com
WHITE PLAINS, NY, July 11, 2000 — Out of 22 brands of ginseng dietary supplements evaluated by ConsumerLab.com eight were found to contain high levels of specific pesticides, some of which also contained significant levels of lead.
News Release
April 24, 2000
ConsumerLab.com announces first test-based buyer's guide to dietary supplements; Manufacturers encouraged to have products tested for potential inclusion.
WHITE PLAINS, NY, April 24, 2000 — ConsumerLab.com announced today that it is developing the first consumer guide to dietary supplements to be based on comprehensive independent laboratory testing of popular supplements sold in the U.S. The ConsumerLab.
News Release
January 31, 2000
Quality of prostate supplements get mixed review in ConsumerLab.com study; Saw palmetto product review published online today.
WHITE PLAINS, NY, January 31, 2000 — Out of 27 major brands of dietary supplements purporting to contain saw palmetto for treating symptoms of prostate enlargement, only 17 appeared to have ingredients similar to those found in published clinical studies.
Clinical Update
9/12/2023
Ginkgo for Tinnitus?
Do ginkgo supplements reduce symptoms of tinnitus? Find out what recent studies show in the What It Does section of our Ginkgo Supplements Review.
Also see: Do any supplements or lifestyle changes reduce the symptoms of tinnitus?
CL Answer
With herbal supplements, what is the difference between root powder and root extract? Does it matter?
Learn more about the different forms of herbal supplements, including aloe, boswellia, ginkgo, green tea, and milk thistle.
Clinical Update
1/11/2022
Ginkgo and Cistanche for Chronic Fatigue Syndrome?
Does supplementing with ginkgo and cistanche extracts help reduce symptoms of chronic fatigue syndrome? See what a recent study showed in our updated answer to the question: Which supplements help to improve energy and decrease fatigue?
News Release
January 24, 2000
Majority of dietary supplements sold in U.S. being tested by ConsumerLab.com in 2000; newest results due January 31 for Saw Palmetto.
WHITE PLAINS, NY, January 24, 2000 — As part of its ongoing independent evaluation of health, wellness, and nutrition products sold in the U.S., ConsumerLab.com announced that by year-end it is to have independently tested most of the popular brands of dietary supplements sold in the U.S.
News Release
December 08, 1999
ConsumerLab.com testing quality of Glucosamine, Chondroitin, and Saw Palmetto supplements; web site becoming popular reference for consumers
WHITE PLAINS, NY, December 8, 1999 — As part of its ongoing independent evaluation of health, wellness, and nutrition products sold in the U.S., ConsumerLab.com announced that it is now testing Glucosamine, Chondroitin, and Saw Palmetto dietary supplements.
Recalls & Warnings
October 27, 2004
Some Supplements Can Damage Eyes At High Doses and with Topical Use
As reported by Reuters Health on October 21, 2004, an article in the current issue of the American Journal of Ophthalmology suggests that some herbal remedies and nutritional supplements can damage the eyes.
Recalls & Warnings
January 20, 2006
Another Ginkgo Supplement Recalled
On January 19, 2006, Olympian Labs informed ConsumerLab.com that it recently issued a recall of a line of Ginkgo biloba supplements because the products "did not meet our specifications that our raw material supplier was contracted to follow.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
May 07, 2013
FDA Warns Consumers Of Three More Sexual Enhancement Supplements Containing Undeclared Drugs
On May 7, 2013, the FDA issued a warning to consumers, urging them not to purchase or use three sexual enhancement products which were found to contain undeclared drugs.
Recalls & Warnings
January 17, 2017
Seller of Heart, Senior and Teen Supplements Warned For Drug Claims
On December 7, 2016, the FDA issued a warning letter to Esteem Products Ltd following a review of the company's website, which found statements made about its products, Cardio Life, Total Man, Total Woman, Senior Total Man, Senior Total Woman , and Total Teen to be drug ...
Recalls & Warnings
August 13, 2016
Seller of Omega-3, Ginkgo, Vision Supplements & More Warned for Drug Claims
On July 22, 2016, the FDA issued a warning letter to Viva Nutraceuticals (J & E International Corporation) following a review of the company's website and promotional literature, which found statement made about its products, Eyestonia, Ginkgo Biloba 60mg, Golden Omega-3 Max, ...
Recalls & Warnings
February 19, 2014
Seller of Sexual Enhancement Supplements Warned For Manufacturing Violations
On February 11, 2014, the FDA issued a warning letter to Maximus Niterider International Group, Inc.
Recalls & Warnings
June 03, 2019
Vinpocetine May Cause Miscarriage or Fetal Harm
Women who are pregnant or who could become pregnant should not take Vinpocetine because it may cause miscarriage or harm fetal development, the FDA warned in a news release today.
Recalls & Warnings
May 21, 2014
Seller of Gingko and Milk Thistle Warned for Manufacturing Violations and Drug Claims
On April 4, 2014, the FDA issued a warning letter to Xtra Life Natural Systems, Inc.
Recalls & Warnings
March 22, 2016
NOW Foods Recalls Ginkgo & Other Supplements
On March 18, 2016, NOW Foods issued a recall of the following dietary supplements due to errors on the labels:
Recalls & Warnings
August 16, 2013
Ginkgo, Milk Thistle, Cleanse and Nopal Supplement Maker Warned For Manufacturing Violations, Drug Claims
On July 23, 2013, the FDA issued a warning letter to Natural Products Services, Inc.
Recalls & Warnings
October 24, 2012
FDA Seizes Many Supplements from New York Company Due To Drug Claims
On October 23, 2012, U.S. Marshalls, acting on behalf of the FDA, seized dietary supplements and unapproved drugs from Confidence, Inc., a supplement manufacturer in Port Washington, N.Y. The products included dietary supplements Dr.
News Release
November 16, 1999
Only three quarters of Ginkgo Biloba supplements contain proper ingredients. ConsumerLab.com posts first of its dietary supplement product tests.
WHITE PLAINS, NY, November 16, 1999 — Consumers have a one out of four chance of buying a Ginkgo Biloba product that may not deliver the ingredients they are seeking. This is the finding of an independent analysis of products conducted by ConsumerLab.
Recalls & Warnings
September 16, 2016
Memory Enhancement Ingredient Vinpocetine Should Not Be Sold In Supplements, Says FDA
On September 7, 2016, the FDA announced its tentative conclusion that the synthetic compound vinpocetine, currently sold in some dietary supplements, should not be classified as a dietary supplement ingredient.
Recalls & Warnings
October 14, 2015
No Vinpocetine in Some Vinpocetine Supplements; Picamilon Labels Not Accurate
A recently published study of 23 supplements listing vinpocetine as an ingredient found six did not contain any vinpocetine. Among those that did contain vinpocetine, most did not list amounts on the label, but amounts found in suggested daily servings varied by almost 100-fold, from 0.
Recalls & Warnings
June 20, 2003
Mixing Herbal Supplements, Migraine Medications Can Be Dangerous
On June 19, 2003, ABC News reported than an extensive review of existing research has led University of Utah researchers to conclude several popular herbal supplements may cause adverse, even deadly, reactions when combined with certain migraine medications.
Recalls & Warnings
September 08, 2011
FDA Warns Several Supplement Manufacturers Not Following Good Manufacturing Practices
In recent weeks, the U.S. FDA has sent warning letters to several supplement manufacturers regarding deficiencies identified during audits of their facilities and not adequately corrected. Use the links below to access the Warning Letters on the FDA website.
Recalls & Warnings
February 03, 2015
Major Retailers Accused of Selling Adulterated Herbal Supplements
On February 3, 2015, the New York State Attorney General announced that his office sent letters to four major retailers, GNC, Target, Walmart, and Walgreens, for allegedly selling store brand herbal supplement products in New York that either could not be verified to contain the labeled substance, ...
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
December 21, 2012
Weight Loss Supplement Containing Undeclared Drug Recalled
On December 19, 2012, P&J Trading issued a voluntary recall of SLIMDIA REVOLUTION after FDA testing found the dietary supplement contains undeclared sibutramine.
Recalls & Warnings
May 03, 2013
Recall: Two Sexual Enhancement Supplements Containing Undeclared Drugs
On May 1, 2013, American Lifestyle issued a voluntary recall of all lots of sexual enhancement supplements Vicerex and Black Ant because they were found to contain undeclared prescription drugs.
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
April 04, 2013
Three More Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On April 3, 2013, the FDA warned consumers that the sexual enhancement supplements AFFIRM XL, Love Rider and Ninja Mojo were found to contain undeclared erectile dysfunction drugs. Consumers are urged to stop using these supplements immediately.
Recalls & Warnings
June 13, 2013
Seller of Cardio, Immune, Greens Supplements and More Warned For Drug Claims
On May 29, 2013, the FDA issued a warning letter to dietary supplement company Nature's Answer, following a review a the company's website, www.naturesanswer.
Recalls & Warnings
February 21, 2013
FDA and FTC Warn: Supplements Cannot Prevent, Treat Or Cure Cold And Flu
On February 11, 2013, the FDA and the Federal Trade Commission (FTC) jointly issued warning letters to three dietary supplement companies regarding their potential illegal marketing of products to prevent, treat or cure flu virus.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
April 27, 2023
Seller of Magtein, Veggie Caps, & More Warned for Drug Claims, Manufacturing Violations
On March 8, 2023, the FDA issued a warning letter to Spartan Enterprises Inc., dba Watershed Wellness Center, after a review of the company’s website found statements about its Dr. Bob’s Naturals Spirulina, Dr. Bob’s Naturals Magtein, Dr.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
September 12, 2024
Root Bioscience Warned for CBD Claims
On August 30, 2024, the FDA issued a warning letter to Root Bioscience Brands, LLC dba Naternal following a review of the company's websites, which found statements made about the company's products, including CBD Oils Move CBD+CBG Oil, Rest CBD+CBN Oil, Nurture Broad Spectrum ...
Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Recalls & Warnings
February 06, 2023
Recalled Eye Drops Linked to Vision Loss and One Death
On February 2, 2023, Global Pharma Healthcare issued a nationwide recall of its Artificial Tears Lubricant Eye Drops due to potential contamination with bacteria that can cause serious infection, blindness, and death.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Recalls & Warnings
February 17, 2022
Seller of L-Theanine, Lutein, Noopept and More Warned for Drug Claims
On February 4, 2022, the FDA issued a warning letter to Crystal Clear Supplements following a review of the company’s website and social media which found statements about Tianeptine Sodium Powder, Tianeptine Sulfate Powder, PEA Capsules, Phenibut Capsules, Synephrine Powder, Noopept ...
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
August 28, 2018
FDA Warns Seller of Goldenseal and Parasite Supplements
On August 15, 2018, the FDA issued a warning letter to Healthy Healing dba Crystal Star, following a facility inspection which found certain statements product labels, including product labels for Women's Best Friend and Para Purge, to be drug claims.
Recalls & Warnings
October 16, 2014
Seller of Black Cohosh, Ginkgo and More Warned For Manufacturing Violations and Drug Claims
On April 2, 2014, the FDA issued a warning letter to Pure Herbs, Ltd.
Recalls & Warnings
September 16, 2015
Herbal Extracts Recalled
On September 15, 2015, Iowa Select Herbs, LLC issued a recall of numerous herbal exacts following a permanent injunction which required the company to stop selling supplements.
Recalls & Warnings
December 16, 2013
Weight Loss Capsules Recalled Due Allergen Risk
On December 13, 2013, Stone Independent Research, Inc. issued recall of one lot of Zanocap Scientific Weight Loss 500 mg capsules because they contain undeclared whey protein, which is derived from milk.
Recalls & Warnings
May 10, 2013
Seller Of Sexual Enhancement and Hormonal Balance Supplements Warned For Manufacturing Violations
On April 26, 2013, the FDA issued a warning letter to Pristine Bay, L.L.C.
Recalls & Warnings
December 16, 2015
Maker of Red Yeast Rice, St. John's Wort, Valerian and More Warned for Manufacturing Violations, Drug Claims
On December 2, 2015, the FDA issued a warning letter to Nature's Health, LLC, following a facility inspection which found the company's product, including Ginkgo & Rhodiola, Blood Sugar Balance IV, Cinnamon Extract, Choles-Balance Red Yeast Extract, Lecithin, Milk Thistle Seed Extract, ...
Recalls & Warnings
September 08, 2017
Probiotic for Infants Recalled Due to Potential Choking Hazard
On September 7, 2017, Garden of Life, LLC, issued a recall of Baby Organic Liquid, a liquid probiotic supplement for infants, because the product, as labeled, includes directions for use that may be misinterpreted and could pose a choking hazard due to the thickness of the liquid.
Recalls & Warnings
November 14, 2012
Mushroom Supplement Manufacturer Warned For Drug Claims and Adulteration
On October 18, 2012, the FDA issued a warning letter to Mushroom Wisdom, Inc. following a facility inspection and product label review which found the dietary supplements Amyloban 3399 From Lion's Mane, Grifron Maitake, and Super Shiitake to be promoted as drugs.
Recalls & Warnings
February 28, 2013
Maker of Liquid Omega-3 Supplements Warned For Manufacturing Violations
On February 20, 2013, the FDA issued a warning letter to Capco Custom Packaging Inc.
Recalls & Warnings
August 26, 2014
Supplement Maker Warned for Manufacturing Violations
On August 14, 2014, the FDA issued a warning letter to dietary supplement manufacturer Big Easy Confections following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing ...
Recalls & Warnings
July 15, 2014
Pinnacle Labs International Warned for Manufacturing Violations
On June 24, 2014, the FDA issued a warning letter to Pinnacle Labs International, Inc.
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
May 31, 2014
Sellers of "Cancer" Supplements Warned for Drug Claims
Two sellers of graviola supplements promoted to treat cancer have been issued warning letters by the FDA. Graviola (Annona muricata), also known as soursop, is the fruit of a tree native to South America and Puerto Rico.
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
August 08, 2014
FDA Warns Puerto Rico Supplement Seller
On June 30, 2014, the FDA issued a warning letter to Mr. Ramon Rosa, following a review of his website, www.aceitedeguanabana.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
September 26, 2014
FDA Warns Seller of Supplements and Chocolate Promoted to Treat Ebola
On September 23, 2014, the FDA issued a warning letter to Natural Solutions Foundation, following a review of the company's websites which found statements made about Silver Sol Nano Silver (also called The Silver Solution) and CBD Organic Dark Chocolate Bars (also called High Potency CBD Hemp Oil) ...
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
August 26, 2014
Seller of Immune Supplement Warned for Drug Claims
On August 12, 2014, the FDA issued a warning letter to Ad-Med Biotechnology, following a review of the company's websites and product labels, which found statements made about Immune Active Adult Formula and Immune Active Children's Formula to be drug claims.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
October 24, 2012
Warning Issued to Memory Supplement Maker For Unapproved Drug Ingredient, Drug Claims and Unreported Adverse Events
On October 16, 2012, the FDA issued a letter to dietary supplement manufacturer Quincy Bioscience Manufacturing Inc.
Recalls & Warnings
June 07, 2017
FDA Warns Seller of Probiotic and Omega-3 Supplements for Manufacturing Violations
On May 22, 2017, the FDA issued a warning letter to BioTE Medical, LLC following a facility inspection which found its products, including BioTE DIM, BiotTE Probiotic, BioTE Iodine Plus, and BioTE Omega 3 to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
February 14, 2017
Life Extension Warned for Drug Claims
On February 1, 2017, the FDA issued a warning letter to Life Extension Foundation Buyers Club, Inc.
Recalls & Warnings
January 21, 2017
Seller of "Cancer Kits," Alpha Lipoic Acid Supplements and More Warned for Drug Claims
On December 22, 2016, the FDA issued a warning letter to Northern Health Products, Inc. following a review of the company's websites, www.northernhealthproducts.com and www.petdca.
Recalls & Warnings
August 06, 2016
Some Supplements May Cause or Exacerbate Heart Failure (Includes vitamin E and many herbs)
Taking certain supplements may be dangerous for people with heart failure, according to a statement from the American Heart Association (AHA) which was published in the journal Circulation (August 2, 2016).
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
August 23, 2015
Maker of Herbal Supplements Sold on eBay, Amazon and Other Websites Shut Down
On August 17, 2015, a federal court ordered a permanent injunction against dietary supplement company Iowa Select Herbs LLC for unlawfully manufacturing and distributing unapproved new drugs and misbranded drugs and adulterated and misbranded dietary supplements.
Recalls & Warnings
August 11, 2015
Company Ordered to Stop Making and Selling Supplements
On August 4, 2015, a federal court ordered a permanent injunction against dietary supplement company Atrium Inc., and two companies under the same ownerhship, Aspen Group Inc., Nutri-Pak of Wisconsin Inc.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
May 30, 2015
Seller of Supplements for Herpes, Prostate Cancer & More Warned for Drug Claims
On May 7, 2015, the FDA issued a warning letter to Strictly Health Corporation, following a review of the company's websites which found statements made about FENVIR, Prosta Pep and Tonalin brand CLA to be drug claims.
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
February 24, 2015
Seller of Heart, Brain and Diabetes Supplements Warned for Drug Claims
On February 6, 2015, the FDA issued a warning letter to LCW, Inc.
Recalls & Warnings
February 10, 2015
Seller of "Natural" Cough Syrup Warned for Manufacturing Violations, Drug Claims
On January 21, 2015, the FDA issued a warning letter to Fragrance Manufacturing Incorporated, following a facility inspection which found the company's products, including Maty's All Natural Cough Syrup and Maty's All Natural Cough Syrup for Kids, to be adulterated because they ...
Recalls & Warnings
November 18, 2015
Seller of "Hangover" Supplement Warned for Manufacturing Violations & Drug Claims
On September 17, 2015, the FDA issued a warning letter to Life Support Development Ltd, following a facility inspection which found the company's product, including Life Support Hangover Relief to be adulterated because they prepared, packed, or held under conditions that violate ...
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
November 11, 2014
Maker of Children's Vitamins, Weight Loss Supplements and More Warned for Manufacturing Violations
On October 30, 2014, the FDA issued a warning letter to VitalHealth Tech, Inc., following a facility inspection which found the company's products, including Kids Mighty Vites tablets, Omni Jr.
Recalls & Warnings
November 11, 2014
Seller of Sexual, Muscle Enhancement Supplements Warned for Manufacturing Violations and Drug Claims
On August 26, 2014, the FDA issued a warning letter to GE Pharma LLC following a facility inspection which found the company's products, including Fire Burn, Fire Storm, Creatine, Amino Fire, Nitric Fire, Jet Fire, Oxy Fire, HGH, Cissus, Hydro shield, Performa-Test, Raspberry Ketones, Fire Drol, ...
Recalls & Warnings
October 31, 2014
Seller of "Cleanse," Allergy Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 15, 2014, the FDA issued a warning letter to Evangelical Christian Ministries, dba Health & Herbs, following a facility inspection which found the company's products, CORYDALIS and CVF BGONE to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
October 30, 2014
Supplement Maker Warned for Manufacturing Violations
On October 21, 2014, the FDA issued a warning letter to DNG Trading & Milling, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
October 21, 2014
Seller of Weight Loss Supplements Warned for Manufacturing Violations and Drug Claims
On October 7, 2014, the FDA issued a warning letter to YoungYou International, Inc.
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
January 17, 2015
Supplement Maker Warned for Manufacturing Violations
On August 5, 2014, the FDA issued a warning letter to Engineering Nutrition, following a facility inspection which found the company's products, which were not named, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for ...
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
December 12, 2014
Seller of Omega-3 "Syrup" Warned for Manufacturing Violations and Drug Claims
On October 16, 2014, the FDA issued a warning letter to Mr.
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
January 03, 2012
Recall by Eclectic Institute of Supplements Containing Gotu Kola and Bladderwrack
On December 22, 2011, the U.S.






