Reviews and Information for Eden
Search term may appear only in full report available to members. Join now for full access.
Product Review
Gluten-Free Grains Review (Buckwheat, Quinoa, Sorghum, Millet, and Teff)
31% of Gluten-Free Grains Fail Our Tests Due to Contaminants. Quinoa, Buckwheat, Sorghum, Millet and Teff Tested.

Product Review
Pumpkin Seeds (Pepitas) Review
We Identified the Best Pumpkin Seeds

Product Review
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
Recalls & Warnings
July 19, 2023
FDA Warns Seller of Sleepy Time Products and Honey Herbal Syrups for Manufacturing Violations
On June 28, 2023, the FDA issued a warning letter to Eden’s Answers, Inc. after an inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
CL Answer
I've heard that soaking dried beans for 24 hours reduces the phytate level, allowing for greater access to nutrients. Is this true?
Does soaking dried beans in water help reduce phytate levels to help gain greater access to nutrients?

CL Answer
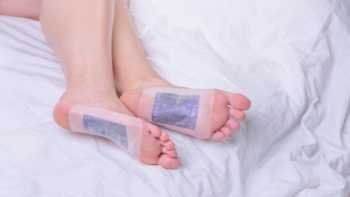
Do detox foot pads or ionic foot baths remove heavy metals or other toxins from the body?
Detox foot pads and ionic foot baths are promoted for removing heavy metals and other toxins from the body. Find out if there is evidence to support these claims.
Recalls & Warnings
December 18, 2006
Canada Warns of Herbal Sleep Product Containing Rx Drug
On December 18, 2006 Health Canada advised consumers not to use a product called Eden Herbal Formulations Sleep Ease Dietary Supplement, because it was found to contain an undeclared drug estazolam, which can be habit-forming when used for as little as a few months.
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 21, 2011
FDA Seizes Probiotics Over Marketing Claims
On June 7, 2011, the U.S. FDA announced that, at its request, U.S. Marshals seized probiotic products from UAS Laboratories, Inc., of Eden Prairie, Minn. because the company markets the products as drugs.





