Product Reviews and Information for Digestive Support
Search term may appear only in full report available to members. Join now for full access.
Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

CL Answer
Are Hormone Harmony and other Happy Mammoth Supplements Worth Taking?
Are the supplements Hormone Harmony, Hormone Harmony Plus+, Prebiotic Collagen Protein, or Bloat Banisher by Happy Mammoth likely to be beneficial for menopause symptoms or digestive health? Find out.

CL Answer
Have you heard of the probiotic, Keybiotics? Does it do what it claims, and is it worth the money they charge?
Whole Body Research Keybiotics probiotic information, including its ingredients and possible cheaper alternatives.

CL Answer
Since the COVID-19 drug, Paxlovid, works by blocking a protease enzyme, should I be concerned about taking digestive enzymes that contain proteases?
Find out if taking digestive enzyme supplements that contain proteases might interact with medications that work by blocking a protease enzyme, including the COVID-19 drug Paxlovid.
CL Answer
What are the benefits of Daily Synbiotic by Seed Health? Does it do what it claims, and is it better than other probiotics and prebiotics?
Review of Daily Synbiotic by Seed, including information about the prebiotic and probiotic strains in the supplement, and whether there is evidence of benefit for the synbiotic.

CL Answer
Do any supplements help with pancreatitis? Do any cause pancreatitis?
Find out if supplements including digestive enzymes, antioxidants, or medium-chain triglycerides are beneficial for chronic pancreatitis, and learn which supplements might worsen symptoms.
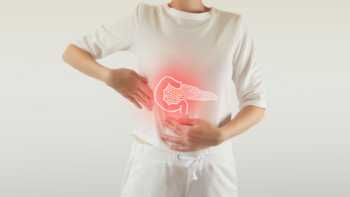
CL Answer
Which supplements, foods or diet and lifestyle changes help relieve acid reflux (heartburn), and which worsen it?
Find out which supplements and foods can help relieve acid reflux, and which can make it worse.
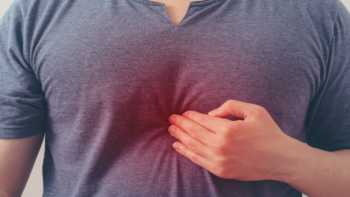
CL Answer
Can zuPOO and its primary ingredient, cascara sagrada, cleanse the gut, improve digestion and help with weight management?
zuPOO is marketed for gut support and as a colon cleanser for weight management. Find out if research supports these claims and if ingredients in zuPOO are safe.

CL Answer
What is Skinny Fiber and does it really work? I see that glucomannan is a key ingredient. What is that?
Learn more about Skinny Fiber, including its key ingredient glucomannan, evidence from clinical studies, dosage info, and safety.

CL Answer
Do sea moss and carrageenan have health benefits? Are there risks?
Sea moss is promoted for thyroid health, boosting immunity, improving gut health, and other uses. Find out if it works and learn about possible side effects of sea moss and its constituent, carrageenan.

CL Answer
Can heat and humidity destroy whey protein and other sports nutrition supplements (e.g., creatine, BCAAs, digestive enzymes) during shipping or while storing at home?
Find out if heat and humidity can degrade creatine, BCAAs, digestive enzymes, and other muscle enhancing supplements.

CL Answer
Do Any Supplements Improve Dry Mouth (Xerostomia) or Make It Worse?
Find out if any supplements, topical formulations (patches, rinses, lozenges, toothpastes, sprays, gels or gums), or lifestyle modifications reduce dry mouths symptoms.

CL Answer
Do any supplements help for irritable bowel syndrome (IBS)?
IBS supplements may help reduce irritable bowel symptoms such as pain, diarrhea and constipation. Supplements include probiotics, melatonin, and flaxseed.

CL Answer
Which supplements or OTC medications help reduce flatulence (gas), and are there any that make it worse?
Find out which supplements may help reduce flatulence (gas), and learn which supplements or foods may worsen symptoms of flatulence.

CL Answer
Titanium dioxide is listed as an ingredient in my supplement. Is it safe?
Titanium dioxide in supplements might have you concerned. Learn more about titanium dioxide - FDA status and evidence from studies on cancer.

CL Answer
Which supplements are important after bariatric surgery (i.e., weight loss or stomach-reducing surgery)? Are there any I should avoid?
Find out which supplements may help after weight loss or stomach reducing surgery, including iron and calcium, and which should be avoided.
CL Answer
Is it true that low-carb or Mediterranean diets help for gastroesophageal reflux disease (GERD)?
Find out if a low-carb diet, such as the Mediterranean diet, or lifestyle changes help with gastroesophageal reflux disease (GERD).

CL Answer
Adrenal Fatigue and Adrenal Support Supplements: Do they work and are they safe?
Find out what's really in supplements for adrenal support and adrenal fatigue, including herbal adrenal support supplements and bovine derived adrenal support supplements. Find out if they really work and if they are safe. ConsumerLab.com's answer explains.

CL Answer
Does Restore (Biomic Sciences LLC) really improve gut health? What is in Restore?
Learn more about Restore, including clinical studies on gut health, dosage, cost, and safety.

CL Answer
What are the health benefits of serrapeptase?
Serrapeptase is a proteolytic enzyme. Learn more about its health effects, including whether evidence supports its use for pain, inflammation, and heart health.

Clinical Update
12/15/2018
Enzymes for Indigestion
Will taking digestive enzymes reduce indigestion? Although not all studies have shown a benefit, a recent study suggested improvements with no adverse effects. For details, see the Digestive Discomfort section of the Digestive Enzyme Supplements Review. (Also see our top choices for enzyme supplements).
Clinical Update
6/03/2022
Digestive Enzymes for IBS?
Does taking digestive enzymes reduce symptoms of irritable bowel syndrome (IBS)? Find out in our updated Digestive Enzyme Supplements Review.
CL Answer
I take omeprazole (Prilosec), a proton pump inhibitor, to reduce stomach acid. Are there supplements I should avoid, or be taking, due to this drug?
Supplement interactions with proton pump inhibitor (PPI) drugs like omeprazole (Prilosec) and Nexium.

CL Answer
Which supplements and foods help relieve constipation and which can cause constipation?
Find out which supplements can cause constipation, including iron, calcium, protein powders and drinks and others. ConsumerLab.com's answer explains.

CL Answer
Mullein Tea: Possible Health Effects and Safety
Mullein tea is often promoted for relieving symptoms of lung conditions, including coughing, hoarseness, sore throat, and shortness of breath. Find out if there is evidence to support these uses, and learn about possible side effects of mullein.

CL Answer
How do I choose the best probiotic supplement? There are so many different strains of bacteria!
Information on how to choose the best probiotic supplement based on safety and our lab test on probiotic bacteria content.

Clinical Update
11/28/2023
Enzymes for Eye Floaters
Does supplementing with digestive enzymes reduce eye floaters and improve sight? See what a recent study showed in the Eye Floaters section of our Digestive Enzymes Supplements Review.
Also see: Do any supplements help prevent or reduce eye floaters?
Clinical Update
3/24/2023
Bromelain Supplements on Amazon Fail Tests
Only four of 20 brands of bromelain purchased on Amazon provided the enzyme activity expected from their labels, three of which had no detectable enzyme activity, according to a recent report. For details by brand, see the Quality Concerns section of our Digestive Enzyme Supplements Review, which includes our Top Picks among digestive enzyme products.
Clinical Update
12/06/2022
Digestive Enzymes – Prescription vs. Supplements
A CL member with chronic pancreatitis asked about alternatives to costly prescription enzymes. See our Digestive Enzyme Supplements Review for information about prescription enzymes as well as supplements similar to prescription products.
CL Answer
Is Greek yogurt a good source of protein?
Find out if Greek yogurt is a good source of protein, how much protein it contains, and what type of protein it contains..

CL Answer
Is there evidence that d-limonene can help fight cancer?
Find out if d-limonene can help fight cancer or help for GERD (gastroesophageal reflux disorder), including evidence from clinical studies.

CL Answer
Butyrate Supplements for Gut Health: Does butyric acid really work and is it safe?
Can butyrate supplements improve gut health or help reduce symptoms of irritable bowel syndrome (IBS) or Crohn's disease? Find out what the clinical evidence shows, learn about the different forms of butyrate, including sodium butyrate, calcium-magnesium butyrate, and tributyrate, and get details about products such as BodyBio Sodium Butyrate and Healus Complete Biotic Tributyrin, dosage, safety and cost.

CL Answer
Does AZO Bladder Control really work for overactive bladder?
AZO Bladder Control information, including results from clinical studies on bladder control, dosage, and safety.

CL Answer
What are the health benefits of amla and is it safe?
Learn about amla (Indian gooseberry), including its potential health benefits and possible safety concerns.

CL Answer
Do any supplements help prevent or treat a cold?
Are there supplements that can help prevent or treat cold symptoms? Learn more about zinc, probiotics, vitamin C and more.

Recalls & Warnings
February 04, 2020
Seller of Digestion Supplements Warned for Manufacturing Violations
On January 10, 2020, the FDA issued a warning letter to Marco Pharma International LLC, which found the company's Absinthium Herbal Liquid Extract 100 ml (Absinthium), promoted for "digestive support", and other products, including S21 Multi Somaplex 100 ml (Multi Somaplex), ...
CL Answer
Can turmeric or curcumin with black pepper extract cause constipation? I had this problem, but it resolved when I switched to a curcumin supplement without piperine (black pepper extract).
Learn more about black pepper extract, why it is added to turmeric/curcumin supplements, and if it can cause gastrointestinal effects.

CL Answer
Can eating almonds reduce wrinkles or have other health benefits?
Find out if eating almonds can reduce wrinkles or improve aging skin.

CL Answer
What is the ingredient zeolite I see in Natural Cellular Defense and other detox supplements? Does it help in any way?
Learn what zeolite is and why it is found in many detox supplements like Waiora's Natural Cellular Defense, Get Healthy Again Zeolite, and Ultra Liquid Zeolite.

CL Answer
How can I find out where a vitamin or supplement is made and where its ingredients come from, such as China or the USA?
Learn more about the manufacturing, distribution, and ingredients of a supplement, including FDA regulations.

CL Answer
Black Pepper Extract & Turmeric: Is black pepper extract necessary for turmeric to be effective, and is it safe?
Find out the best way to take turmeric and curcumin supplements to increase absorption and bioavailability. ConsumerLab.com's answer explains.

CL Answer
Is P-5-P really better than "regular" vitamin B6?
Learn more about pyridoxal-5-phosphate (P-5-P) and vitamin B6.

CL Answer
Why is beta-carotene still an ingredient in some supplements? Isn't it dangerous?
Beta-carotene can be found in supplements. Get clinical evidence from studies on beta-carotene's impact on mortality, smoking, and vision.

Clinical Update
9/29/2023
Supplements for Pancreatitis?
Can digestive enzyme supplements work as well as prescription enzymes for chronic pancreatitis, and can any other supplements help? Find out, plus, learn if any supplements can cause pancreatitis, in our new article about supplements and pancreatitis.
Clinical Update
4/13/2019
Does Vitamin D Improve Cancer Survival?
Does giving high-dose vitamin D to people with cancer of the colon or other parts of the digestive tract improve survival rates? Find out what recent studies are showing in the What It Does section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D supplements.
Clinical Update
9/04/2018
Glutamine for IBS?
Glutamine, an amino acid, plays a role in maintaining the digestive tract. Can taking it as a supplement help people with irritable bowel syndrome (IBS)? Find out what a recent study showed in the Glutamine section of the Muscle Enhancers Review.
Clinical Update
1/14/2017
Enzymes for Muscle Soreness
A blend of enzymes reduced delayed-onset muscle soreness after exercise in healthy men. For details, see the "What They Do" section of the Digestive Enzyme Supplements Review >>
Clinical Update
8/07/2021
Curcumin for Indigestion?
How well does curcumin (from turmeric) improve digestive symptoms such as indigestion, diarrhea or constipation? See what a new study found in the What It Does section of our Turmeric and Curcumin Supplements & Spices Review. Also see our Top Picks among turmeric and curcumin products.
Clinical Update
9/21/2021
Enzyme Supplements for Eye Floaters?
Can enzyme supplements (bromelain, papain and ficin) reduce eye floaters? Find out what a recent study suggests in the What They Do section of our Digestive Enzyme Supplements Review.
Also see our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
8/12/2024
High Blood Pressure from "Liver Support" Supplement
A woman developed high blood pressure after daily use of a “liver support” supplement. Find out the likely reason why in our article about licorice root tea and supplements.
News Release
September 26, 2024
What’s Really In Shilajit Supplements? ConsumerLab Tests Reveal Amounts of Fulvic Acid & Heavy Metals
White Plains, NY, September 26, 2024 -- Shilajit is a tar-like substance created by decomposed plant material and found in mountain rocks.
News Release
November 10, 2022
Best Probiotic Supplements Identified by ConsumerLab Tests
White Plains, New York, November 10, 2022 — Probiotic supplements can be helpful in preventing or treating constipation, diarrhea, flatulence, vaginal infections, and other conditions, but with so many different products on the market, choosing the best one is no easy task.
News Release
April 14, 2020
ConsumerLab Tests Reveal Best Probiotic Supplements and Those With Quality Issues
White Plains, New York, April 14, 2020 — Probiotic supplements may be helpful for conditions including antibiotic-associated diarrhea, irritable bowel syndrome, and respiratory infections.
News Release
May 15, 2019
ConsumerLab Tests Reveal Big Differences in Digestive Enzyme Supplements
White Plains, New York, May 15, 2019 — Digestive enzyme supplements can help improve digestion and the absorption of nutrients, and may reduce symptoms of indigestion. But to work, they must provide a certain amount of enzyme activity.
Clinical Update
10/19/2021
Mixing Boosters for J&J Recipients?
Find out why some experts support mixing boosters for J&J vaccine recipients. Also see new research indicating that an mRNA vaccine may be the best booster for those already given a J&J-type vaccine.
Clinical Update
8/02/2015
Prebiotics for Immune Health
Prebiotics are complex sugars which can support the growth of probiotic organisms in the gut. A recent study reported a specific type of prebiotic increased amounts of beneficial bacteria and improved certain markers of immune function in older men and women. Get the details, including dosage, plus our tests of popular probiotics and prebiotics in the Probiotic Supplements Review >>
Clinical Update
6/29/2019
Popular Probiotic -- What's In a Name?
Does the popular probiotic VSL#3 contain a different formula than used in the clinical studies supporting its benefits? Find out in the What to Consider When Buying section of the Probiotics Supplements Review. Also see our Top Picks for probiotic supplements.
Clinical Update
6/20/2018
Intestinal Benefit of Cocoa?
Cocoa flavanols may support intestinal health, according to an animal study with a cocoa powder that acted like a prebiotic. Get the details in the What It Does section of the Cocoa and Dark Chocolate Review. (Also see our Top Picks among cocoa and dark chocolate products.)
Clinical Update
12/10/2024
Prevagen Must Cease Memory Claims
A U.S. District Judge recently ordered the marketers of Prevagen – promoted for memory -- to cease the use of eight marketing claims due to a lack of scientific support. For details, see our article about Prevagen.
Clinical Update
10/18/2024
Wellness Formula for Colds?
The product Wellness Formula by Source Naturals is said to support the immune system to help people avoid sick days. Is there evidence of that? Find out in our article about supplements for colds.
Also see: Do any supplements help for flu?
Clinical Update
9/12/2023
Sea Moss
Sea moss, sometimes called Irish moss, is promoted for improving thyroid health, boosting immunity, and improving gut health, but are these uses supported by clinical evidence? Find out in our article about sea moss and its constituent carrageenan.
Clinical Update
10/20/2023
Osha Root for Immune Support?
Osha root is promoted to reduce congestion and coughs, boost the immune system, and more. But does it work, and is it safe? Find out in our article about the potential benefits and side effects of osha root.
Clinical Update
1/12/2024
Pricey Toothpaste for Gum Health?
Tartar Control Fluoride Toothpaste by Smileactives is promoted as “supporting healthy gums” but is it worth $36 per tube? Find out in the Preventing bleeding gums section of our article about toothpastes and other dental products, which includes our Top Picks among products for bleeding gums, preventing cavities, desensitization, dry mouth, and bad breath.
Clinical Update
3/19/2024
Probiotic for COVID?
Did a probiotic promoted to support immune defenses against COVID-19 help prevent or treat COVID-19? Find out in our Probiotics Supplements Review, which includes our Top Picks among probiotics.
Also see: Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Clinical Update
4/09/2024
Vegamour for Hair?
Vegamour GRO Hair Serum is marketed for making hair thicker and fuller but is there any clinical evidence to support this claim? Find out in our article about supplements for hair.
Clinical Update
1/20/2025
Ionic Foot Bath Claims
Ionic foot baths (such as IonCleanse and Healifeco Ionic Foot Spa) allegedly draw heavy metals out of the body. But is this supported by evidence? Find out in our article about foot pads and foot baths for removing toxins from the body.
Clinical Update
1/23/2025
Dose For Your Liver?
The supplement Dose For Your Liver is promoted for improving liver health and signs of fatty liver disease, but does research support this? Find out in our article about supplements and diets for fatty liver.
Clinical Update
4/10/2025
Does H2TAB Work?
We took a look at a list of studies that supposedly support the use of H2TAB hydrogen water tablets. See our take on these studies in our article about hydrogen water.
Clinical Update
6/17/2022
L-carnosine for Cardio Fitness?
Does research support L-carnosine supplementation for improving cardio fitness? Find out what a study showed among people with heart failure in our updated CL Answer about L-carnosine.
Clinical Update
6/06/2023
Berberine's New Popularity
Berberine is being called "nature’s Ozempic" – referring to the type 2 diabetes drug also used for weight management. But is there evidence to support this? See our Berberine Review to find out what it can do, if it's safe, how products rate on quality, and our top choices.
Clinical Update
7/18/2023
Aspartame & Cancer
A group of experts recently classified aspartame as "possibly carcinogenic." Learn what evidence was used to support this classification, and find out if all experts agree with it, in the Aspartame section of our article about sugar substitutes.
Clinical Update
5/09/2023
Prostadine for Prostate & Urinary Health?
Prostadine is a supplement that claims to support prostate health and bladder control, but does it really work? Find out in our Prostate Supplements Review, which includes our Top Pick among prostate supplements.
Also see our article about supplements to reduce nighttime urination.
Clinical Update
12/06/2022
Hair Supplement Benefits?
Does research support the use of hair supplements such as Forti5, Lambdapil, or Nourkrin for treating thinning of hair? Find out in our updated CL Answer about supplements, topicals and medications for hair.
Clinical Update
8/23/2022
Can-C Eye Drops for Dry Eye?
A product called Can-C Eye Drops has been promoted for improving dry eye as well as reducing the risk of cataracts. Does the evidence support this? Find in our updated CL Answer about supplements for dry eye.
Clinical Update
9/30/2022
Does MSM Help Hair and Nails?
Find out if research supports MSM (methylsulfonylmethane) for improving nails or hair in the MSM section of our Joint Health Supplements Review.
Also see our article about biotin and other supplements for hair and nails.
Clinical Update
10/07/2022
Biotin for Skin Health?
Biotin has been promoted for reducing wrinkles, but does clinical research support this? Find out in the Biotin (B-7) section of our B Vitamin Supplements Review, which also discusses the evidence for biotin for treating brittle nails and hair loss.
Also, see our CL Answer about supplements for reducing wrinkles and tightening the skin.
Clinical Update
1/13/2023
Lifestyle Changes for Tinnitus?
Find out if research supports the following lifestyle changes for reducing symptoms of tinnitus:
Clinical Update
2/17/2023
TUDCA for Fatty Liver?
TUDCA supplements are commonly promoted for treating liver disorders, but is there evidence supporting the use of TUDCA for fatty liver disease? Find out in our updated CL Answer about supplements for fatty liver.
Also, see our CL Answer about TUDCA for details about its other uses, potential safety concerns, and cost.
Clinical Update
2/28/2023
TUDCA for Acid Reflux?
A CL Member with acid reflux reported symptom relief with TUDCA (tauroursodeoxycholic acid) supplementation, but does clinical evidence support this use? Find out in our updated CL Answer about TUDCA.
Also, see our CL Answer about supplements that may improve or worsen acid reflux.
Recalls & Warnings
October 12, 2013
Metagenics Enzyme Supplement Recalled Due To Drug Risk
On October 11, 2013, Metagenics Canada issued a voluntary recall of digestive enzyme supplement Spectrazyme, due to potential contamination with the prescription antibiotic chloramphenicol.
Recalls & Warnings
February 05, 2014
Maker of Digestive Health Supplement Warned For Manufacturing Violations, Drug Claims
On January 22, 2014, the FDA issued a warning letter to Intensive Nutrition Incorporated, following a facility inspection which found the company's digestive support supplement, Tanalbit, to be adulterated because it was prepared, packed, or held under conditions that violate Current Good ...
News Release
March 11, 2019
Best NAC (N-acetyl cysteine) Supplements Identified by ConsumerLab
White Plains, New York, March 11, 2019 — NAC (N-acetyl cysteine) supplements are promoted for many uses, including "liver support," "immune support," and reducing symptoms of the flu and flare-ups of chronic bronchitis.
News Release
May 02, 2018
Best Probiotic Supplements and Kombuchas? -- ConsumerLab Tests 41 Popular Products, Reveals Problems and Top Picks
White Plains, New York, May 2, 2018 — With so many different probiotic supplements on the market, each providing different amounts and strains of beneficial bacteria or yeast, choosing the right one can be an overwhelming task.
Recalls & Warnings
January 12, 2021
Seller of Cholesterol, Weight Loss Products, and More Warned for Claims
On November 17, 2020, the FDA issued a warning letter to Bonagens following a review of the company's website, which found statements made about the company's products Anti-Aging Supports, Cholesterol & Diabetes Control, Gout Control, Hypertension Control, Liver Supports, Lungs ...
News Release
February 24, 2017
Vitamin D Supplements Maintain Top Spot in Popularity, as Probiotics Surpass Multivitamins to #4 Behind Fish Oil and CoQ10 in Latest ConsumerLab.com Survey
White Plains, New York, February 24, 2017 — A recent survey of over 9,505 people who use dietary supplements shows the most popular dietary supplement to be vitamin D, followed by fish oil, CoQ10, probiotics, and multivitamins.
News Release
November 06, 2015
Some Surprising Results from Tests of 43 Probiotic Supplements and Kefir Drinks
White Plains, New York, November 6, 2015 — How many beneficial organisms are in probiotic supplements and kefir drinks? Are any contaminated with pathogens, such as E. coli, and do those labeled as "gluten free" or "99% lactose-free" live up to their claims? To find out, ConsumerLab.
News Release
May 05, 2015
ConsumerLab.com's Tests of Digestive Enzyme Supplements Reveal What Labels Don't
White Plains, New York, May 5, 2015 — How do you know if your digestive enzyme supplement is truly active? The only way to know for sure is to test it on fats, carbohydrates, proteins and other parts of food which it is expected to digest. That's exactly what ConsumerLab.
News Release
November 25, 2013
Many Probiotic Supplements Fall Short on Listed Amounts of Helpful Organisms
White Plains, New York, November 25, 2013 — Probiotic supplements and foods containing "friendly" bacteria or yeast have become popular among people hoping to improve bowel function, immunity, and even mood.
Recalls & Warnings
April 25, 2024
Yogi Tea Recalled Due to Pesticides
On April 19, 2024, Yogi Tea issued a recall of over 54,800 packs of Yogi Echinacea Immune Support tea after a sample of echinacea tested above acceptable limits for multiple pesticides. No illnesses have been reported to date.
Recalls & Warnings
December 26, 2024
Sleep Supplement Recalled Due to Undeclared Soy
On October 30, 2024, Deseret Biologicals Inc. recalled 2,547 bottles of DesBio lunaSOMM Natural Sleep Support Dietary Supplement capsules, which are labeled as containing phosphatidylserine from sunflower lecithin powder but contain undeclared soy lecithin.
Recalls & Warnings
December 15, 2020
FDA Warns Seller of Evening Primrose Oil and Beta Glucan
On December 2, 2020, the FDA issued a warning letter to Smoky Mountain Naturals, LLC following a review of the company's website, which found statements made about the company's products Evening Primrose Oil and Beta Glucan to be drug claims.
Recalls & Warnings
January 20, 2021
Seller of Omega-3 Warned for Making Claims to Treat Focus, Mood
On December 9, 2020, the FDA issued a warning letter to Bodyhealth.com, LLC following a review of the company's website, which found statements made about the company's products Healthy-Thin Energize, Body Detox (Oral Spray), and Omega 3 Health to be drug claims.
Recalls & Warnings
March 10, 2025
Probiotics and Prebiotic Gummies Recalled
On January 17, 2025, Health and Happiness (H&H) LLC.
Recalls & Warnings
November 10, 2020
FDA Warns Seller of Digestive Enzymes, Nattokinase, and More
On October 27, 2020, the FDA issued a warning letter to World Nutrition, Inc.
Recalls & Warnings
December 10, 2024
Aroma Vita Hot Cocoa Mix Recalled Due to Metal Fragments
On October 2, 2024, Dyma Brands Inc. issued a recall of two lots of Aroma Vita Hot Cocoa Mix, because they may contain metal fragments which, in severe cases, could lead to damage of the digestive tract if ingested.
Recalls & Warnings
November 06, 2023
FDA Warns Consumers Not to Use Dr. Ergin’s SugarMD Advanced Glucose Support, Found to Contain Prescription Drugs
On November 3, 2023, the FDA warned consumers not to purchase or use Dr. Ergin’s SugarMD Advanced Glucose Support products after FDA laboratory analysis found the supplement to contain glyburide and metformin.
Recalls & Warnings
December 26, 2024
Blood Sugar Supplement Recalled Due to Undeclared Metformin and Glyburide
On December 16, 2024, Shoppers- Plaza recalled all lots of Fouzee Sugarlin Herbal Formula capsules because they were found to contain undeclared metformin and glyburide.
News Release
January 31, 2013
Use of CoQ10, Digestive Enzymes, Probiotics and B Vitamins on the Rise According to ConsumerLab.com Survey
White Plains, New York — February 1, 2013 — A recent survey of over 10,000 people who take supplements shows that CoQ10, digestive enzymes, probiotics, and B vitamins are the four categories experiencing the most growth over the prior year.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Recalls & Warnings
August 03, 2023
Ozona Organics Liquid Probiotics Recalled Due to Risk of Microbial Contamination
On August 1, 2023, Ozona Organics, LLC recalled certain lots of its probiotic supplement, Ozona Probiotics for Digestive Health, also labeled as GoHealthy Probiotics for Infants, Toddlers, and Kids and GoHealthy Probiotics for Infants, Kids, Men, and Women, due to the ...
Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
News Release
August 31, 2012
ConsumerLab.com tests selenium supplements -- Finds one with less than 25% of listed amount
White Plains, New York, August 31, 2012 — ConsumerLab.com announced results today of its Product Review of Selenium Supplements. Among the supplements selected by ConsumerLab.com for testing, one was found to contain only 23.
Recalls & Warnings
August 11, 2022
Problems With Supplements on Amazon
More than 50% of 30 top-listed immune support supplements purchased on Amazon.com in May of last year were found to list ingredients that could not be found in them with testing, according to a recent report (Crawford, JAMA Network Open, 2022).
Recalls & Warnings
March 01, 2023
Seller of Dr. Miller’s Herbal “Detox” Teas Warned by FDA for Drug Claims
On February 3, 2023, the FDA issued a warning letter to Jackson Health & Wellness Clinic after inspection of the company’s website found statements about the company’s Dr. Miller’s Holy Tea, Dr. Miller’s Super Holy Tea, Dr. Miller’s Ultimate Tea, Dr.
Recalls & Warnings
March 23, 2023
FDA Warns Herbal Vitality for Promoting Supplements to Treat Cold & Flu, Kidney Stones & More
On March 7, 2023, the FDA issued a warning letter to Herbal Vitality, Inc.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
July 31, 2020
FDA Warns Seven Sellers of "Hangover Cures"
The FDA recently issued warning letters to seven companies for promoting hangover relief products with drug claims (use the links below to read the full warning letter):
Recalls & Warnings
September 10, 2021
FDA Warns Ten Sellers of "Diabetes" Supplements
On September 7, 2021, the FDA issued warning letters to 10 supplement companies that made drug claims by promoting products to treat diabetes and/or lower blood sugar. Five of the products were sold on Amazon as well as on company websites. The products were promoted with statements such as
Recalls & Warnings
August 08, 2022
Seller of CBD Warned for COVID-19 Claims
On August 4, 2022, the FDA sent a warning letter to FluxxLab LLC following a review of the company’s website and social media which found statements about the company’s Covid-19 Immune Support Tincture and CBDA+CBD Oil Tincture products to be drug claims because they ...
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
July 03, 2012
Standard Process Recalls Three Supplements Due to Possible Salmonella Contamination
On June 29, 2012, Standard Process issued a voluntary recall of the company's Cataplex ACP, Cataplex C and Pancreatrophin PMG, after a routine FDA inspection uncovered potential Salmonella contamination in an ingredient found in these three products.
Recalls & Warnings
November 18, 2015
Seller of "Hangover" Supplement Warned for Manufacturing Violations & Drug Claims
On September 17, 2015, the FDA issued a warning letter to Life Support Development Ltd, following a facility inspection which found the company's product, including Life Support Hangover Relief to be adulterated because they prepared, packed, or held under conditions that violate ...
Recalls & Warnings
February 16, 2021
Seller of BioCBD+ Products Warned for COVID-19 and Drug Claims
On February 11, 2021, the FDA issued a warning letter to Evolved Ayurvedic Discoveries, Inc.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
March 04, 2022
FTC, FDA, and DOJ Take Joint Action Against Herbal Tea Companies for COVID Claims
On March 3, 2022, the FDA, FTC, and DOJ sued a New York-based marketer of herbal tea, in attempts to permanently block deceptive ads that claim Earth Tea is clinically proven to treat, cure, and prevent COVID-19.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
September 25, 2018
FDA Warns Seller of Digestive Enzyme Supplements
On March 6, 2018, the FDA issued a warning letter to Uckele Health & Nutrition, Inc.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Joint and Hair Loss Products
On December 22, 2020, the FDA issued a warning letter to Speedwinds Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Synotrex and Sephren to be drug claims.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Turmeric, Ashwagandha, Glucosamine, and More
On December 18, 2020, the FDA issued a warning letter to Reddy Naturals following a review of the company's website, which found statements made about the company's products Glycostasis, Glucosamine Curcumin, Turmeric 50, Turmeric Curcumin, Cetyl Curcumin, Ashwagandha, Huruli, ...
Recalls & Warnings
January 18, 2002
Recall of Pepsin-containing Digestive Supplements
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 16, 2002 Enforcement Report.
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
May 31, 2014
Sellers of "Cancer" Supplements Warned for Drug Claims
Two sellers of graviola supplements promoted to treat cancer have been issued warning letters by the FDA. Graviola (Annona muricata), also known as soursop, is the fruit of a tree native to South America and Puerto Rico.
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
May 30, 2015
Seller of Supplements for Herpes, Prostate Cancer & More Warned for Drug Claims
On May 7, 2015, the FDA issued a warning letter to Strictly Health Corporation, following a review of the company's websites which found statements made about FENVIR, Prosta Pep and Tonalin brand CLA to be drug claims.
Recalls & Warnings
June 07, 2012
Recall of Digestive Health Supplement Due to Salmonella Concern
On June 4, 2012, Botanical Laboratories of Ferndale, WA announced the recall of 38 bottles of 33.8 oz Wellese "Digestive 3 in 1 Health " liquid dietary supplement and 275 bottles of 16 oz.
Recalls & Warnings
April 03, 2020
FDA Warns Pure Plant Essentials for Essential Oil Coronavirus Claims
On April 1, 2020, the FDA issued a warning letter to Health Mastery Systems DBA Pure Plant Essentials for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
December 15, 2020
Mushroom Supplement Seller Warned for Drug Claims
On December 4, 2020, the FDA issued a warning letter to Desert Alchemist LLC following a review of the company's website, social media, and Etsy.com store (www.etsy.
Recalls & Warnings
September 08, 2020
Seller of "Diabetes Supplement" & More Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Nutritional Supplements Corporation Inc following a review of the company's website, which found statements made about some of the company's products, including Vitadone, Diabrex, and Viadevita to be drug claims.
Recalls & Warnings
April 24, 2021
Metal Reported in Church & Dwight Gummy Melatonin, Multis & Fiber Supplements
On April 20, 2021, Church & Dwight Co., Inc.
Recalls & Warnings
October 11, 2013
Prebiotic and Digestive Enzyme Supplement Recalled Due To Drug Risk
On October 10, 2013, Adeeva Nutritionals Inc. issued a voluntary recall of digestive supplement Flora Essentials due to potential contamination with the prescription antibiotic chloramphenicol.
Recalls & Warnings
April 11, 2014
Digestive Health Supplement Recalled Due to Salmonella Risk
On April 4, 2014 Swanson Health Products issued a voluntary recall of digestive health supplement Swanson Premium Brand Full Spectrum Cilantro (Coriander), because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
June 16, 2019
"Immune Support" Throat Sprays Recalled
On June 13 2019, APS BioGroup, Inc. issued a recall of four "immune support" throat sprays because they have the potential to be contaminated with Stenotrophomonas maltophilia, a bacteria that can cause respiratory infection, particularly in individuals with weakened immune systems.
Recalls & Warnings
June 05, 2014
More Chia Powders Recalled Due to Salmonella Risk
On June 4, 2014, Health Matters America Inc. issued a voluntary recall its Organic Traditions Sprouted Chia Seed Powder and Organic Traditions Sprouted Chia & Flax Seed Powder because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
May 29, 2014
Chia Powders and Smoothies Recalled Due to Salmonella Risk
On May 28, 2014, Navitas Naturals issued a voluntary recall of products containing its organic sprouted chia seed powder because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
November 01, 2013
Protein Shakes Containing Prescription Drug Pose Serious Health Risk
On November 1, 2013, Health Canada warned consumers that Vega One Vanilla Chai (sizes 437g and 874g) and Vega Sport Performance Chocolate nutritional shakes, by Sequel Naturals Ltd., have been found to contain the prescription antibiotic chloramphenicol.
Recalls & Warnings
September 07, 2012
Prebiotics Recalled For Salmonella Risk
On September 4, 2012, Eco Health, Inc. announced a recall of its florAlign Prebiotic Formula (Powder) due to the potential for the product to be contaminated with Salmonella.
Recalls & Warnings
April 23, 2013
Seller of Digestion, Thyroid and Vitamin D Supplements Warned For Drug Claims
On November 5, 2012, the FDA issued a warning letter to The Women's Health Institute at Texas following a review of the company's various websites which found statements made about Digest + SEB, Iodine Plus 2, and D5000 Vitamin D dietary supplements to be drug claims.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
January 11, 2012
Herbal Extract Company Warned by FDA of Making Drug Claims
On January 3, 2012, the U.S. FDA sent a Warning Letter to Herbal Extract Plus, LLC regarding legal violations for claiming its herbal products can cure or prevent diseases.
Recalls & Warnings
December 23, 2017
Seller of Meal Replacement, Protein Drinks, Cranberry and More Warned for Manufacturing Violations
On July 6, 2017, the FDA issued a warning letter to Professional Botanicals, Inc.
Recalls & Warnings
August 26, 2014
Supplement Maker Warned for Manufacturing Violations
On August 14, 2014, the FDA issued a warning letter to dietary supplement manufacturer Big Easy Confections following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing ...
Recalls & Warnings
July 15, 2014
Pinnacle Labs International Warned for Manufacturing Violations
On June 24, 2014, the FDA issued a warning letter to Pinnacle Labs International, Inc.
Recalls & Warnings
June 01, 2012
Homeopathic Manufacturer Settles False Advertising Suit
June 1, 2012 — A federal court in California recently authorized the settlement of a class action lawsuit against Boiron Inc. and Boiron USA, Inc.
Recalls & Warnings
August 28, 2013
Tea and Papaya Supplement Company Warned For Drug Claims
On August 12, 2013, the FDA issued a warning letter to Herbal Papaya, LLC, following a review of the company's website and Facebook page which found statements made about Papaya Seed Extract Capsules, 100% Papaya Leaf (Paw Paw Twig) and Papaya Leaf with Rooibos Tea to be drug claims.
Recalls & Warnings
September 10, 2012
Maker of Concussion Recovery Supplement Warned For Making Drug Claims
On August 28, 2012, the FDA issued a warning letter to Trinity Sports Group, Inc. after a review of the company's websites found statements about Neuro Impact Concussion Response Formula constituted drug claims not permitted on supplements.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
February 13, 2019
Supplements Promoted for Alzheimer's Disease and Dementia Sell "False Hope," Warns FDA
On February 11, 2019, the FDA warned consumers to beware supplements promoted to prevent or treat Alzheimer's disease or dementia.
Recalls & Warnings
June 05, 2018
FDA Warns Seller of Colloidal Silver
On May 17, 2018, the FDA issued a warning letter to Silver Armor, Inc.
Recalls & Warnings
June 06, 2017
Seller of Herbal Supplements Warned for Manufacturing Violations, Drug Claims
On May 25, 2017 the FDA issued a warning letter to Life Rising Corporation, following a facility inspection which found the company's products, including Stomach Regulator, Fang Feng Formula, Skin Regulator, Regulate Liver, Circulation Regulator, Pancreas Support, Lung Regulator, Pure Tea, ...
Recalls & Warnings
July 06, 2012
Centrum Multivitamins: Breast and Colon Health Claims Pulled
On July 5, 2012, Pfizer Consumer Healthcare announced it will withdraw breast and colon health claims from its Centrum multivitamin advertising and labels, and will revise health and energy claims made on other Centrum products.
Recalls & Warnings
January 16, 2016
Seller of Liver, Lung Support Supplements Warned for Drug Claims
On January 4, 2016, the FDA issued a warning letter to Tibetan Herbal Balance, Inc.
Recalls & Warnings
October 01, 2016
No Evidence Supplement Can Reverse or Prevent Gray Hair, Says FTC
On September 23, 2016, the FTC announced a U.S. district court has issued a judgement prohibiting the makers of Grey Defence dietary supplements from making claims the products can reverse or prevent grey hair, unless they are not misleading and are supported by reliable scientific evidence.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
October 05, 2012
Illegal Claims on Weight Loss, Immune System Dietary Supplements, and Few Backed by Clinical Data
On October 3, 2012, the U.S. Department of Health and Human Services issued a report warning that dozens of weight loss and immune system supplements have illegal claims of curing or treating disease on their labels and/or lack the scientific evidence to support such label claims.
Recalls & Warnings
November 17, 2005
FTC Warns Web Sites Peddling Hormone Replacement
On November 10, 2005, the Federal Trade Commission (FTC) announced that its staff had sent warning letters to 34 Web site operators making claims that products advertised as natural alternatives to hormone replacement therapy will prevent or treat diseases, such as cancer, heart disease, or ...
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
Recalls & Warnings
January 18, 2013
FTC Upholds Ruling, POM Wonderful Health Claims Were Deceptive
On January 16, 2013, the Federal Trade Commission (FTC) announced it will uphold a judge’s ruling that makers of POM Wonderful 100% Pomegranate Juice and POMx supplements made deceptive and unsubstantiated claims that the products could treat, prevent, or reduce the risk of heart disease, prostate ...
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
July 18, 2017
Seller of "Super Strength Prostate Formula," Acai Berry Supplements & More Warned for Drug Claims
On June 30, 2017, the FDA issued a warning letter to Nature's Health Company, LLC, following a facility inspection and review of the company's website, www.natureshealthcompany.
Recalls & Warnings
May 20, 2015
Supplements to Eliminate Gray Hair Not Supported by Science, Says FTC
On May 13, 2015, the FTC announced marketers of GetAwayGrey and Rise-N-Shine, dietary supplements promoted to reverse or prevent gray hair, have agreed to settle charges that claims made about the products are not supported by science.
Recalls & Warnings
December 12, 2014
Marketers of HCG Settle FTC Charges of Deceptive Weight Loss Claims
Marketers of HCG Platinum drops have agreed to pay $1 million to settle Federal Trade Commission (FTC) charges that claims the drops could cause rapid and substantial weight loss were deceptive and not supported by scientific evidence.
Recalls & Warnings
February 06, 2015
POM Wonderful Claims Were Deceptive, Court Rules
On January 30, 2015, the Federal Trade Commission (FTC) announced that an appeals court has upheld the Commission's ruling that marketers for POM Wonderful made deceptive health claims about the product.
Recalls & Warnings
November 14, 2012
Mushroom Supplement Manufacturer Warned For Drug Claims and Adulteration
On October 18, 2012, the FDA issued a warning letter to Mushroom Wisdom, Inc. following a facility inspection and product label review which found the dietary supplements Amyloban 3399 From Lion's Mane, Grifron Maitake, and Super Shiitake to be promoted as drugs.
Recalls & Warnings
February 07, 2002
Recall of Additional Pepsin-containing Digestive Aids
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its February 6, 2002 Enforcement Report.
Recalls & Warnings
November 21, 2012
Maker of Blood Pressure, Probiotic, Resveratrol and Hormonal Supplements Warned For Manufacturing Violations and Drug Claims
On November 2, 2012, the FDA issued a warning letter to Atrium, Inc. following a facility inspection which found a number of the company's dietary supplements to be adulterated or promoted as drugs.
Recalls & Warnings
May 29, 2013
Seller of Colloidal Silver, Noni Juice and Noni Supplements Warned For Drug Claims
On May 16, 2013, the FDA issued a warning letter to Matrix Health Products, Inc.
Recalls & Warnings
September 05, 2013
Seller of Herbal Supplements and "Tonics" Warned For Manufacturing Violations and Drug Claims
On May 24, 2013, the FDA issued a warning letter to Sundial Herbal Products following a facility inspection which found the company's products, including Woman Back Tonic, Koromantee, Wood and Root Tonic, Arthritis, Asthma, Diabetics, Sundial Ashanti Weight Loss Energy Lifter, Appetite Suppresser, ...
Recalls & Warnings
May 21, 2014
Seller of Gingko and Milk Thistle Warned for Manufacturing Violations and Drug Claims
On April 4, 2014, the FDA issued a warning letter to Xtra Life Natural Systems, Inc.
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
Recalls & Warnings
April 17, 2018
FDA Warns Seller of Echinacea, Iron, Aloe Supplements & More for Manufacturing Violations
On March 30, 2018, the FDA issued a warning letter to Ozark Country Herbs following a facility inspection which found a number of the company's products, including Echinacea-Goldenseal, Natural Iron, and Aloe-Vera Goldenseal Salve, to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
January 17, 2015
Supplement Maker Warned for Manufacturing Violations
On August 5, 2014, the FDA issued a warning letter to Engineering Nutrition, following a facility inspection which found the company's products, which were not named, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for ...
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
December 12, 2014
Seller of Omega-3 "Syrup" Warned for Manufacturing Violations and Drug Claims
On October 16, 2014, the FDA issued a warning letter to Mr.
Recalls & Warnings
February 10, 2015
Seller of "Natural" Cough Syrup Warned for Manufacturing Violations, Drug Claims
On January 21, 2015, the FDA issued a warning letter to Fragrance Manufacturing Incorporated, following a facility inspection which found the company's products, including Maty's All Natural Cough Syrup and Maty's All Natural Cough Syrup for Kids, to be adulterated because they ...
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
November 11, 2014
Maker of Children's Vitamins, Weight Loss Supplements and More Warned for Manufacturing Violations
On October 30, 2014, the FDA issued a warning letter to VitalHealth Tech, Inc., following a facility inspection which found the company's products, including Kids Mighty Vites tablets, Omni Jr.
Recalls & Warnings
November 11, 2014
Seller of Sexual, Muscle Enhancement Supplements Warned for Manufacturing Violations and Drug Claims
On August 26, 2014, the FDA issued a warning letter to GE Pharma LLC following a facility inspection which found the company's products, including Fire Burn, Fire Storm, Creatine, Amino Fire, Nitric Fire, Jet Fire, Oxy Fire, HGH, Cissus, Hydro shield, Performa-Test, Raspberry Ketones, Fire Drol, ...
Recalls & Warnings
August 26, 2014
Seller of Immune Supplement Warned for Drug Claims
On August 12, 2014, the FDA issued a warning letter to Ad-Med Biotechnology, following a review of the company's websites and product labels, which found statements made about Immune Active Adult Formula and Immune Active Children's Formula to be drug claims.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
August 08, 2014
FDA Warns Puerto Rico Supplement Seller
On June 30, 2014, the FDA issued a warning letter to Mr. Ramon Rosa, following a review of his website, www.aceitedeguanabana.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
October 21, 2014
Seller of Weight Loss Supplements Warned for Manufacturing Violations and Drug Claims
On October 7, 2014, the FDA issued a warning letter to YoungYou International, Inc.
Recalls & Warnings
October 16, 2014
Seller of Black Cohosh, Ginkgo and More Warned For Manufacturing Violations and Drug Claims
On April 2, 2014, the FDA issued a warning letter to Pure Herbs, Ltd.
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
October 31, 2014
Seller of "Cleanse," Allergy Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 15, 2014, the FDA issued a warning letter to Evangelical Christian Ministries, dba Health & Herbs, following a facility inspection which found the company's products, CORYDALIS and CVF BGONE to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
October 30, 2014
Supplement Maker Warned for Manufacturing Violations
On October 21, 2014, the FDA issued a warning letter to DNG Trading & Milling, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
September 26, 2014
FDA Warns Seller of Supplements and Chocolate Promoted to Treat Ebola
On September 23, 2014, the FDA issued a warning letter to Natural Solutions Foundation, following a review of the company's websites which found statements made about Silver Sol Nano Silver (also called The Silver Solution) and CBD Organic Dark Chocolate Bars (also called High Potency CBD Hemp Oil) ...
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
February 24, 2015
Seller of Heart, Brain and Diabetes Supplements Warned for Drug Claims
On February 6, 2015, the FDA issued a warning letter to LCW, Inc.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
August 23, 2015
Maker of Herbal Supplements Sold on eBay, Amazon and Other Websites Shut Down
On August 17, 2015, a federal court ordered a permanent injunction against dietary supplement company Iowa Select Herbs LLC for unlawfully manufacturing and distributing unapproved new drugs and misbranded drugs and adulterated and misbranded dietary supplements.
Recalls & Warnings
August 11, 2015
Company Ordered to Stop Making and Selling Supplements
On August 4, 2015, a federal court ordered a permanent injunction against dietary supplement company Atrium Inc., and two companies under the same ownerhship, Aspen Group Inc., Nutri-Pak of Wisconsin Inc.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
February 28, 2017
Multivitamin Recalled Due to Allergen Risk
On February 14, 2017, Licata Enterprises issued a recall of all lots of its Supreme One and Theravits 100 multiple vitamins because they contain fish liver oil but is labeled as containing "no common allergens.
Recalls & Warnings
October 31, 2017
Black Licorice Can Cause Abnormal Heart Rhythms, FDA Warns
On October 30, 2017, the FDA warned consumers that eating 2 ounces of black licorice a day for at least two weeks could cause an irregular heart rhythm or arrhythmia in adults age 40 or older.
Recalls & Warnings
March 29, 2016
Maker of Bone Health, Prostate, Cholesterol Supplements and More Warned for Drug Claims
On March 17, 2016, the FDA issued a warning letter to Rx Vitamins, Inc., following a facility inspection which found the company's products, includingTestost-Rx, DB-7, The Bone Density Formula, NaturLo Cholesterol, ThyRx-7 and Arth-9 to be misbranded.
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
May 20, 2005
Seller of "Ocular Nutrition" Supplement Settles FTC Charges
On March 16, 2005, The Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular Nutrition) – ...
Recalls & Warnings
February 19, 2005
Seller of Eye Supplement Purporting to Restore Vision and Eliminate "Floaters" Settles FTC Charges
On February 15, 2005, the Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, have settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular ...
Recalls & Warnings
February 21, 2013
FDA and FTC Warn: Supplements Cannot Prevent, Treat Or Cure Cold And Flu
On February 11, 2013, the FDA and the Federal Trade Commission (FTC) jointly issued warning letters to three dietary supplement companies regarding their potential illegal marketing of products to prevent, treat or cure flu virus.
Recalls & Warnings
October 06, 2004
False Claims Made by Marketer of Cortisol-related Weight-Loss Supplements According to FTC
On October 6, 2004, the Federal Trade Commission (FTC)charged marketers of two dietary supplements with claiming, falsely and without substantiation, that their products can cause weight loss and reduce the risk of, or prevent, serious health conditions.
Recalls & Warnings
April 09, 2006
Sellers of Children’s Weight-Loss Product Settle FTC Charges
On April 6, 2006, the Federal Trade Commission (FTC) announced that the marketers of Pedia Loss, a purported children’s weight-loss product, and Fabulously Feminine, a supposed female libido enhancement product, had agreed to settle Federal Trade Commission charges that they made false and ...
Recalls & Warnings
October 05, 2011
FDA Warns Herbal Nitro of Manufacturing Violations
The FDA has published a Warning Letter dated September 22, 2011 to Herbal Nitro Inc. regarding manufacturing violations at its facility in Yucaipa, CA.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
June 12, 2014
Marketers of Memory Supplement Settle FTC Charges of Deceptive Claims
Martek Biosciences Corporation and i-Health Inc., marketers of BrainStrong Adult, have agreed to settle Federal Trade Commission (FTC) charges that claims the supplement could improve adult memory and prevent cognitive decline were deceptive, and not supported by sufficient clinical evidence.
Recalls & Warnings
January 16, 2018
Marketers of CellAssure and Cognify Settle FTC Charges of Deceptive Cancer Claims
On January 11, 2018, the FTC announced that CellMark Biopharma, LLC and its CEO, Derek E.
Recalls & Warnings
June 21, 2016
"Doctor Trusted" Seal and Certification Program for Dietary Supplements Misleading and Meaningless, Says FTC
SmartClick Media LLC, also doing business as Doctor Trusted, has agreed to settle Federal Trade Commission (FTC) charges that its "Doctor Trusted" seal and certificates, which appeared on some 800 websites promoting health products and dietary supplements, ...
Recalls & Warnings
October 11, 2016
Seller of Glucosamine and Chondroitin Settles FTC Charges of False Advertising
On October 5, 2016, the FTC announced the sellers of the liquid glucosamine and chondroitin supplement Supple (Supple LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
April 28, 2017
Marketers of Weight Loss System Agree to Settle FTC Charges of Deceptive Claims
On April 21, 2017, the FTC announced the marketers of the NutriMost Ultimate Fat Loss System (NutriMost, LLC and NutriMost Doctors, LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
May 13, 2015
Amberen Weight Loss Claims Not Supported by Evidence, Says FTC
On May 12, 2015, the FTC announced it filed a complaint to stop Lunada Biomedical Inc. from advertising that its product Amberen is clinically proven to cause substantial weight loss in women over 40.
Recalls & Warnings
September 09, 2014
Maker of Popular Green Coffee Bean Extract Settles FTC Charges of Unsupported Weight Loss Claims
Applied Food Sciences, Inc., maker of green coffee bean extract GCA, has agreed to pay $3.5 million to settle FTC charges that weight loss claims made about the extract were not supported by scientific evidence.
Recalls & Warnings
January 27, 2015
Green Coffee Bean Supplement Marketer Settles FTC Charges of Deceptive Claims
On January 26, 2015, the FTC announced that Pure Health LLC, Genesis Today, and owner of both companies, Lindsey Duncan, will pay $9 million to settle charges of deceptive weight loss claims made about the companies' green coffee bean extract products.
Recalls & Warnings
March 10, 2015
Maker of Magnesium, Calcium, Zinc, B Vitamins and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Complete H2O Minerals, following a facility inspection which found the company's products, including Sulfur Concentrate, Copper, Extra Strength Copper, Extra Strength Magnesium, Extra Strength Zinc, Platinum, Gold, Indium, Molybdenum, ...
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
September 05, 2014
Seller of "Nerve", "Cleanse" and Probiotic Supplements Warned for Drug Claims
On July 30, 2014, the FDA issued a warning letter to Plexus Worldwide, Inc., following a review of the company's website and product labels, which found statements made about Fast Relief, BioCleanse and ProBio5 to be drug claims.
Recalls & Warnings
August 02, 2014
Judge Orders BioAnue To Stop Illegal Cancer and Disease Claims
On July 23, 2014, Gloria D.
Recalls & Warnings
September 26, 2014
Sellers of Essential Oils Warned for Claiming to Treat Ebola, Other Diseases
On September 22, 2014, the FDA issued a warning to Young Living following a review of websites, Pinterest, Facebook and Twitter accounts owned by Young Living distributors, which found statements made about the company's essential oils, including Thieves, Cinnamon Bark, Oregano, ImmuPower, ...
Recalls & Warnings
September 26, 2014
Seller Warned for Promoting Essential Oils to Treat Flu, MRSA, Measles and More
On September 22, 2014, the FDA issued a warning to doTERRA International, LLC.
Recalls & Warnings
October 30, 2014
Seller of Vitamin C, Iron and Detox Supplements Warned for Violations, Drug Claims
On October 16, 2014, the FDA issued a warning letter to Vitalab Co., Inc. (which manufactures and labels supplements for three distributors: V.E. Irons, Inc., Springreen Products, Inc., and Sonne's Organic Foods, Inc.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
June 26, 2015
Seller of CoQ10, Anxiety Supplements and More Warned for Manufacturing Violations, Drug Claims
On June 6, 2015, the FDA issued a warning letter to Country Doctor Herbals, following a facility inspection which found the company's products, including Herbal Flu Be-Gone, Herbal Multi-Bac, Herbal Nervine, Herbal Pain-A-Way, Herbal Pancreas, Herbal Parasite Cleanse, Herbal Perfect ...
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
May 30, 2015
Seller of Omega-3, Probiotics & SuperFoods Warned for Manufacturing Violations, Drug Claims
On May 4, 2015, the FDA issued a warning letter to Dr. Dennis Black, LLC.
Recalls & Warnings
December 16, 2015
Maker of Red Yeast Rice, St. John's Wort, Valerian and More Warned for Manufacturing Violations, Drug Claims
On December 2, 2015, the FDA issued a warning letter to Nature's Health, LLC, following a facility inspection which found the company's product, including Ginkgo & Rhodiola, Blood Sugar Balance IV, Cinnamon Extract, Choles-Balance Red Yeast Extract, Lecithin, Milk Thistle Seed Extract, ...
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
August 16, 2013
Ginkgo, Milk Thistle, Cleanse and Nopal Supplement Maker Warned For Manufacturing Violations, Drug Claims
On July 23, 2013, the FDA issued a warning letter to Natural Products Services, Inc.
Recalls & Warnings
January 02, 2012
Manufacturing Violations for Multivitamin, Vitamin K and Other Supplements
On December 19, 2011, the U.S. FDA sent a Warning Letter to Milk Specialties Global regarding manufacturing violations. Affected products include PrimaForce Proliver Capsules, Dr. Mercola Vitamin K2, Active Women’s Multivitamin/Multimineral, Cremagnavol, Immune Support, and HPF Women’s Multi.
Recalls & Warnings
October 05, 2007
FTC Charges Progesterone Cream Sellers with Making Unsubstantiated Claims
On October 5, 2007 the Federal Trade Commission (FTC) announced complaints against seven online sellers of alternative hormone replacement therapy (HRT) products, alleging that they made health claims for their natural progesterone creams without supporting scientific evidence.
Recalls & Warnings
March 15, 2013
Maker of Liquid Supplements Warned For Manufacturing Violations and Drug Claims
On January 31, 2013, the FDA issued a warning letter to Liquid Health, Inc.
Recalls & Warnings
December 01, 2017
Health Research Labs Agrees to Settle FTC Charges of False Claims, Deceptive Marketing of BioTherapex and NeuroPlus
On November 30, 2017, the FTC announced Health Research Laboratories LLC and owner, Kramer Duhon, agreed to settle charges that they made false claims about the company's products, BioTherapex and NeuroPlus.
Recalls & Warnings
April 12, 2013
Adverse Event Reports Associated With Dietary Supplements Increase, But Remain Underreported And Underutilized
On March 18, 2013, the U.S. Government Accountability Office (GAO) announced the number of adverse events reports (AERs) for dietary supplements submitted annually to the FDA has doubled in recent years, increasing from 1,119 in 2008 to 2,480 in 2011.
Recalls & Warnings
June 21, 2002
Canadian Warning on Use of Seven Herbal Supplements from BotanicLab
On June 19, 2002, Health Canada issued a warning that Canadians not use seven herbal products manufactured in the United States by BotanicLab because they contain undeclared prescription drugs that could cause serious health effects if not taken under medical supervision.





