Product Reviews and Information for COVID-19 (Coronavirus)
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Find out if natural remedies & supplements for coronavirus such as zinc, vitamin C, garlic, or elderberry help to prevent or treat COVID-19.
Product Review
Elderberry Supplements Review
Find the Best Elderberry Supplement. Tests and Reviews of Popular Elderberry Supplements & CL's Top Picks.

Product Review
Potassium Supplements Review
Be Careful with Potassium Supplements! Problems Found. Tests and Reviews of Potassium Supplements & CL's Top Picks.
Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

CL Answer
Does Z-Stack multivitamin work to boost the immune system, and is it safe?
Find out about Z-Stack, including if it can help boost the immune system and if it is safe to use at the suggested dose.

Product Review
Vitamin K Supplements Review (Including Calcium, Vitamin D, Magnesium & Boron)
Find Out If It's Worth Taking Vitamin K and Which Products Are Best
Clinical Update
4/08/2022
Andrographis for COVID-19?
Despite early evidence suggesting a benefit, andrographis does not appear to help patients with COVID-19, according to a recent report. Get the details in our updated answer to the question: Do any supplements help with the coronavirus (COVID-19)?
Clinical Update
7/04/2020
Vitamin D & Coronavirus
Studies are linking low blood levels of vitamin D to higher risk of testing positive for the coronavirus and/or developing more severe disease. Find out what blood levels appear to be protective, as well as the results of the most recent study, in the COVID-19 section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
3/25/2020
Surprising Role of Potassium in COVID-19
New research out of China suggests that many patients hospitalized with COVID-19 are low in potassium. For details see the information about Potassium in our answer to the question: What are natural remedies for coronavirus (COVID-19)? Do supplements like zinc, vitamin C, or herbals work?
Clinical Update
6/13/2020
Vitamin D and COVID-19 Risk
Vitamin D deficiency was associated with an increased risk of testing positive for coronavirus according to a recent study. For details see the COVID-19 section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

Clinical Update
2/23/2022
Vitamin D for COVID?
Does high-dose vitamin D help people hospitalized with COVID? See what a recent study showed in the COVID section of our Vitamin D Supplements Review.
Also see our answer to the question: Do any supplements help with the coronavirus (COVID-19)?
Clinical Update
2/04/2022
Vitamin D & Death From COVID
Some research suggests that low blood levels of vitamin D increase the risk of death from COVID, but a new study suggests that high levels also increase risk. Get the details in the COVID section of our Vitamin D Supplements Review.
Also see our answer to the question: Do any supplements help with the coronavirus (COVID-19)?
Clinical Update
9/14/2021
L-Arginine for COVID?
Did L-arginine supplementation help people with severe COVID-related pneumonia? See what a new study found in the What It Does section of our L-Arginine Supplements Review.
Also see: Do any supplements help with the coronavirus (COVID-19)?
Clinical Update
11/11/2021
Sleep, Drink, and COVID Hospitalization Risk
Lack of sleep and frequent consumption of certain beverages may each increase the risk of being hospitalized if you get COVID-19, according to a recent study. Get the details in our updated answer to the question: How are you staying healthy during the coronavirus pandemic?
Clinical Update
7/15/2022
Fraudulent COVID-19 Tests
Several unauthorized COVID antigen tests have been sold recently in the U.S., according to the FDA. Get the details in our answer about testing for coronavirus.
Product Review
L-Lysine Supplements Review
Read L-Lysine Labels Carefully. Some Can Fool You.
Product Review
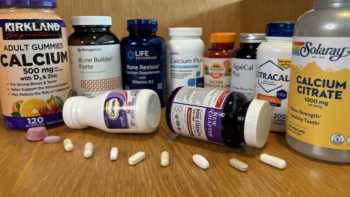
Calcium and Bone Health Supplements Review (Including Vitamins D & K, Magnesium and Boron)
See Which Bone Health Supplements Are Top Picks and Which Fail
Clinical Update
12/30/2021
Hand Sanitizer vs. Soap
How do hand sanitizers or disinfectant hand wipes compare to washing hands with soap and water to remove microbes without causing skin dryness and redness? Find out what a recent study showed in the Hand sanitizer section of our answer to the question: Does heat kill coronavirus (COVID-19)?
Clinical Update
5/17/2022
Omicron Lasts on Surfaces
The Omicron variant of the coronavirus was recently shown to last on surfaces longer than the original (Wuhan) virus. Get the details and possible implications in our answer about COVID-19 disinfection.
CL Answer
Mullein Tea: Possible Health Effects and Safety
Mullein tea is often promoted for relieving symptoms of lung conditions, including coughing, hoarseness, sore throat, and shortness of breath. Find out if there is evidence to support these uses, and learn about possible side effects of mullein.

Product Review
Black Seed Oil Review
Find Out Which Black Seed Oil Supplements Passed or Failed Our Tests. See Our Top Picks.

Product Review
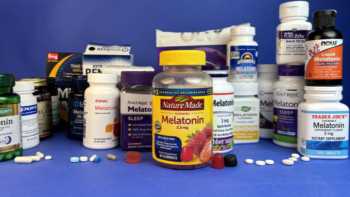
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
CL Answer
What are the health benefits of andrographis? Can it treat colds, help with joint pain, or other conditions, and is it safe?
Learn about the health benefits of andrographis, whether it can help relieve cold symptoms, reduce pain and stiffness of osteoarthritis or rheumatoid arthritis, decrease symptoms of ulcerative colitis, or slow the growth of cancer. See the clinical evidence for the branded andrographis extract ParActin. Plus, learn about dosage, side effects and drug interactions with andrographis.

CL Answer
Which is the best pulse oximeter for home use?
Best pulse oximeter for home use (COVID-19, asthma and COPD). Differences between home use and FDA-approved medical pulse oximeters.

Product Review
Selenium Supplements Review
Choose the Best Selenium Supplement. Find Out If You Need Selenium and Which Supplement Is Our Top Pick.
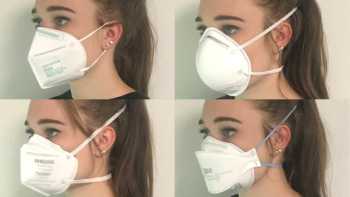
CL Answer
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
Clinical Update
5/09/2023
At-Home COVID Test Recalled
Certain lots of a popular at-home COVID test sold by CVS and Amazon has been recalled due to bacterial contamination. Get the details in our article about testing for coronavirus.
Clinical Update
11/29/2022
When New Boosters Are Most Helpful
The updated Pfizer and Moderna boosters appear to help protect against symptomatic COVID-19, particularly among people who haven’t had a COVID-19 shot in several months, according to a recent study. Get the details in the Updated COVID-19 Boosters section of our CL Answer about COVID-19 vaccines.
Clinical Update
12/16/2023
A Probiotic for COVID-19?
Did supplementing with a popular probiotic reduce the risk of developing COVID-19 symptoms among people exposed to a household member with COVID-19? Find out in the COVID-19 section of our Probiotic Supplements Review, which includes our Top Picks among probiotics.
Clinical Update
3/19/2024
Probiotic for COVID?
Did a probiotic promoted to support immune defenses against COVID-19 help prevent or treat COVID-19? Find out in our Probiotics Supplements Review, which includes our Top Picks among probiotics.
Also see: Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Clinical Update
2/11/2022
Melatonin & COVID
Can supplementing with melatonin help prevent death among people hospitalized with COVID-19? See what a recent study found in the COVID-19 section of our Melatonin Supplements Review, which includes our Top Picks for melatonin.
Also see our answer to the question: Do any supplements help with COVID-19?
Clinical Update
5/20/2022
Gromwell Root for COVID-19?
Can gromwell root (an ingredient in the supplement Tollovid) help treat or prevent COVID-19? See what the latest research shows in our article about supplements for COVID-19.
Clinical Update
6/07/2022
Managing COVID-19 Rebound
Some people experience COVID-19 recurrence after being treated with the antiviral drug Paxlovid. Find out what is recommended when this happens in our updated CL Answer about COVID-19 treatments.
Clinical Update
3/01/2022
COVID-19 and Hair Loss?
Can COVID-19 cause hair loss? Find out in How to You Take Care of Yourself After You’ve Had COVID-19.
Clinical Update
6/02/2020
Vitamin D for COVID-19 Patients
A study evaluated the effects of giving vitamin D, magnesium, and vitamin B12 to COVID-19 patients. See the results in the COVID-19 section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
6/20/2020
Low Potassium in COVID-19 Patients
Patients hospitalized with COVID-19 have often been found to have low potassium levels. Should you be concerned? Learn more in the COVID-19 section of the Potassium Supplements Review. Also see our Top Picks among potassium supplements.
Clinical Update
6/09/2020
A Role for Calcium in COVID-19?
A preliminary study suggests a possible role for calcium supplementation in treating COVID-19. See the details in the COVID-19 section of the Calcium Supplements Review. Also see our Top Picks among calcium supplements.
Clinical Update
10/13/2020
Low Vitamin D & COVID-19 Risk
Studies indicate that vitamin D deficiency may increase the risk of COVID-19 infection. The most recent study suggests this risk may be greater among certain ethnic groups. Learn more in the COVID-19 section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
10/27/2020
Vitamin D and COVID-19
Can taking vitamin D after getting sick with COVID-19 reduce the risk of death? Find out in the COVID-19 section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
10/31/2020
Vitamin C for COVID-19?
Does high-dose vitamin C help people critically ill with COVID-19? Find out what a new report showed in the COVID-19 section of our Vitamin C Supplement Review. Also see our Top Picks for vitamin C.
Clinical Update
11/26/2020
Vitamin D for COVID-19
Can high doses of vitamin D help COVID-19 patients recover? Find out what two new studies showed in the COVID-19 section of our Vitamin D Supplements Review. Also, see our Top Picks for vitamin D.
Clinical Update
12/20/2020
Melatonin for COVID-19?
Can melatonin reduce the duration of symptoms in people with COVID-19? Get the details in the COVID-19 section of our Melatonin Supplements Review. Also, see our Top Picks for melatonin.
Clinical Update
1/10/2021
Vitamin D and COVID-19
Many preliminary studies have linked adequate vitamin D levels with reduced risk of COVID-19 or less severe disease. However, a recent study did not find a benefit among people who had been supplementing with amounts generally above the recommended daily intake requirement for vitamin D. See the details in the COVID-19 section of our Vitamin D Supplements Review. Also, see our Top Picks for vitamin D.
Clinical Update
1/13/2021
High-Dose Vitamin C for COVID-19?
Does high-dose vitamin C help in people with severe COVID-19? See what a new study showed in the COVID-19 section of our Vitamin C Supplements Review.
Clinical Update
9/14/2021
COVID Vaccination and Menstruation
Does COVID-19 vaccination affect menstruation? Find out what is known in our article about COVID-19 Vaccines.
Clinical Update
1/18/2022
Probiotics for COVID-19 Symptoms?
Can a probiotic help resolve COVID-19 symptoms? Learn what a recent study showed in the COVID-19 section of our Probiotic Supplements Review.
Clinical Update
11/23/2021
Reduced "Long" COVID-19 Risk with Vaccination
Getting vaccinated before or even after having COVID-19 has been linked with reduced risk of "long" COVID.
Clinical Update
11/22/2022
Paxlovid & COVID-19 Rebound
Does Paxlovid treatment put one at higher risk for COVID-19 rebound than non-treatment? Find out in the Paxlovid section of our article about COVID-19 treatments.
Clinical Update
7/15/2022
New Vaccine Authorized
The FDA has authorized the Novavax COVID-19 vaccine, which will likely be made available next week. Find out how it differs from the other COVID-19 vaccines, learn about its effectiveness against COVID-19, and get details about possible side effects.
Clinical Update
11/01/2024
COVID and Flu Home Tests Compared
There are now 38 different COVID-19 home antigen tests (7 that also test for flu), but we found big differences in how well they detect COVID (ranging from 59.7% to 96.6% sensitivity) and avoid false negatives. See our comparison chart and learn our Top Picks for testing for COVID and COVID/Flu.
Also, every U.S. household is now eligible to get 4 at-home COVID-19 tests. Find out how (unfortunately, you can’t choose the brand).
Clinical Update
9/09/2022
Booster After COVID?
If you recently had COVID, can you delay getting the new booster? Find out in the Updated COVID-19 Boosters section of our CL Answer about COVID-19 vaccines.
Also, find out if the updated boosters are available without cost.
Clinical Update
11/23/2021
COVID-19 Vaccines and Menstruation
Do COVID-19 vaccins affect menstrual periods? Find out what a recent study suggests.
Clinical Update
1/11/2022
COVID-19 and Menstrual Cycles
Do COVID-19 vaccines affect menstrual cycles? Find out what the latest research shows.
Clinical Update
3/01/2022
Melatonin to Prevent COVID?
Does taking melatonin help prevent COVID-19? Find out what a study found in the COVID-19 section of our Melatonin Supplements Review.
Also see our article about Supplements for COVID.
Clinical Update
3/18/2022
Best COVID Vaccine for Breast-Feeding Women?
For women who are breast-feeding, find out which COVID-19 vaccines are most likely to result in antibodies against COVID-19 in breastmilk.
Clinical Update
6/14/2022
Curcumin for COVID?
Does curcumin supplementation reduce symptoms such as cough, sore throat, and shortness of breath in people with mild to moderate COVID-19? See what a recently published study found in the What It Does section of our Turmeric and Curcumin Supplements and Spices Review.
Also see our article about other supplements proposed to help prevent or treat COVID-19.
Clinical Update
2/23/2022
Paxlovid for COVID-19?
How well does Paxlovid reduce risk of hospitalization or death due to COVID-19 in people who are unvaccinated? Find out in our updated answer about COVID Treatments.
Clinical Update
8/03/2021
Tests to Detect COVID Variants?
Can standard COVID-19 tests identify which variant caused an infection? Find out in our updated CL Answer about tests for COVID-19.
Clinical Update
1/15/2021
Vitamin K & Risk of COVID Death
A marker of low vitamin K levels has been linked to a higher risk of death in people with COVID-19, according to a new study. Get the details in the COVID-19 section of our Vitamin K Supplements Review.
Clinical Update
10/13/2020
Zinc and Risk of COVID Death
A recent study found low blood levels of zinc to be associated with a greater chance of severe symptoms and death in COVID-19 patients. Does this mean that people should supplement with zinc or use zinc lozenges? Find out in the COVID-19 section of our Zinc Supplements and Lozenges Review. Also see our Top Picks for zinc supplements and lozenges.
Clinical Update
10/24/2020
High-Dose Vitamin D for COVID?
Elderly nursing home residents given high-dose vitamin D had much better survival rates from COVID-19 than other residents, according to a new study. However, the dose given has potential risks. Get the details in the COVID-19 section of the Vitamin D Supplements Review.
Clinical Update
Clinical Update
Clinical Update
9/16/2022
Caution with Vitamin C for COVID
Taking very high-dose vitamin C doesn’t seem to provide protection against COVID-19 and it may increase the rate of kidney stones, according to recent data. Get the details in the Vitamin C section of our CL Answer about Supplements and COVID-19.
Clinical Update
7/29/2022
Vitamin D for COVID-19?
A recent study suggested that supplementing with high-dose vitamin D could improve clinical outcomes for people hospitalized with COVID-19. Learn more in the COVID section of our Vitamin D Supplements Review.
Clinical Update
6/17/2022
Travel Requirements Updated
The CDC no longer requires that travelers show a negative COVID-19 test or documentation of recovery from COVID-19 before boarding a flight into the U.S. Get the details about what is still recommended in the Travel section of our article about COVID vaccines.
Clinical Update
11/11/2022
Paxlovid May Reduce Risk of Long COVID
Taking Paxlovid after testing positive for COVID-19 reduced the risk of long-term symptoms. For details, see our CL Answer about COVID-19 treatments.
Clinical Update
10/11/2022
Paxlovid COVID Rebound?
A recent study sheds light on what may account for COVID-19 "rebound" among people treated with Paxlovid. Get the details in our updated CL Answer about treatments for COVID-19.
Clinical Update
10/14/2022
Updated Booster for Younger Kids
The updated COVID-19 boosters are now authorized for younger age groups. Get the details in our article about COVID-19 vaccines.
Clinical Update
10/04/2022
Problem With a Preventive Drug
EVUSHELD (tixagevimab with cilgavimab) is the only product authorized for the pre-exposure prevention of COVID-19, but evidence shows it may provide less protection against Omicron variants compared to earlier strains. Get the details in our CL Answer about treatments for COVID-19.
Clinical Update
11/29/2022
When Paxlovid is Most Helpful
Paxlovid can reduce the risk of hospitalization due to COVID-19, but new data indicates for whom it is most helpful. Get the details in our updated CL Answer about medications for preventing or treating COVID-19.
Clinical Update
10/25/2022
Is It Okay to Take Pain Relievers After COVID-19 Vaccination?
A recent study looked at whether taking OTC pain relievers after vaccination reduced antibody responses to the vaccines. See what the study showed in the Can Pain Relievers / Fever Reducers Be Taken to Lessen Side Effects section of our CL Answer about COVID-19 vaccines.
Clinical Update
3/31/2023
Quercetin and Antivirals
Be aware that quercetin might reduce the effectiveness of some antivirals, including an antiviral in Paxlovid used to treat COVID-19. Get the details in the Concerns and Cautions section of our Quercetin Supplements Review, which includes information about other potential interactions with quercetin, as well as our Top Picks among quercetin supplements.
Also see our CL Answer about Supplements for COVID-19.
Clinical Update
6/28/2022
COVID-19 Tests for Sight Impaired
People with blindness or low vision can now order more accessible COVID-19 tests from the government. Get the details.
Clinical Update
7/08/2022
Omicron BA.5 On the Rise
A new Omicron subvariant, BA.5, has become the dominant SARS-CoV-2 strain in the U.S. Find out how well this virus escapes protection from vaccination or previous infection, and learn if it causes more severe COVID-19 infection, in the Omicron section of our CL Answer about COVID-19 vaccines.
Clinical Update
7/12/2022
How to Get Paxlovid Without a Medical Doctor
Pharmacists can now directly prescribe Paxlovid for people with COVID-19, but there are limitations. Find out what you need to bring to the pharmacy to get Paxlovid if you've tested positive and are at risk for severe COVID-19.
Clinical Update
7/12/2022
Reinfection Risks
Learn how soon after COVID-19 you can get infected again with the Omicron BA.5 variant, which is now dominant in the U.S. and, as we have reported, is more transmissible than earlier variants.
Be aware that getting COVID-19 more than once has been associated with increased risk of developing a variety of health problems, according to recent research.
Clinical Update
7/15/2022
Protection Due to Infection?
How well does a previous COVID-19 infection protect against BA.4/BA.5 infection? It depends on which COVID-19 variant caused the initial infection, according to a recent study.
Clinical Update
8/12/2022
How Many Times Should You Test?
After exposure to COVID-19, how many times should you test? It may be more times than you think, according to a recent study. Get the details in the Antigen Tests section of our CL Answer about COVID-19 Testing.
Clinical Update
4/03/2025
Tinnitus & COVID-19 Vaccination
Is tinnitus (ringing in the ears) a common side effect of COVID-19 vaccination? Find out what evidence suggests in our article about tinnitus.
Clinical Update
5/21/2024
Andrographis Health Effects
Find out if andrographis helps with conditions such as cancer, COVID-19, and multiple sclerosis in our article about the health benefits and safety of andrographis.
Also see: Do any supplements reduce symptoms or relapse of multiple sclerosis?
Also see: Do any supplements help with COVID-19?
Clinical Update
8/15/2023
Vitamin D & COVID-19
Did supplementing with vitamin D reduce symptoms or improve outcomes among people hospitalized with COVID-19? Find out what a recent study showed in our Vitamin D Supplements Review, which includes our Top Picks for vitamin D.
Also see if zinc helped in the same study.
Clinical Update
7/18/2020
Selenium & COVID-19
Selenium-deficient people were more likely to die from COVID-19 than those who were not deficient according to a recent study. For details, see the What It Does section of the Selenium Supplements Review. Also see our Top Pick for selenium.
Clinical Update
4/28/2020
A Role for Vitamin K in COVID-19?
A new study showed a link between vitamin K and the development of COVID-19. Learn what this means in the What It Does section of our Vitamin K Supplements Review. Also see our Top Pick among vitamin K supplements.
Clinical Update
6/14/2020
Do Zinc Lozenges Help?
Several individuals diagnosed with COVID-19 began using zinc lozenges after they became sick. Find out if this was reported to help or not in the COVID-19 section of the Zinc Supplements Review. Also see our Top Picks for zinc supplements and lozenges.
Clinical Update
10/20/2020
Melatonin and COVID-19
Can melatonin supplementation reduce the risk of death in people with severe COVID-19? See what a recent study showed in the What It Does section of our Melatonin Supplements Review. Also see our Top Picks for melatonin.
Clinical Update
8/10/2021
Herbal Treatment
Are traditional Chinese medicines, such as astragalus, helpful in treating or preventing COVID-19? Find out what reviews of the research have concluded in our article about Supplements and COVID-19.
Clinical Update
1/21/2022
Rapid COVID-19 Tests At No Charge
People in the U.S. can now order 4 at-home rapid COVID-19 tests at no charge. Get the details.
Clinical Update
1/21/2022
Is Epstein-Barr Virus Reactivated By COVID-19 Vaccines?
Do COVID-19 vaccines cause reactivation of Epstein-Barr virus? Find out.
Clinical Update
6/14/2022
Duration of EVUSHELD Protection
How long does the medicine EVUSHELD protect against COVID-19? Find out what recent data showed in our article about medicines to prevent and treat COVID-19.
Clinical Update
Clinical Update
Clinical Update
8/17/2021
COVID Risk of Death
Which factors increase the risk of death from COVID-19 among adolescents?
Clinical Update
8/10/2021
COVID Home Test Limitations
COVID-19 home tests, like the 15-minute Abbott BinaxNOW, have significant limitations in real-world use according to a recent study.
Clinical Update
9/03/2021
Fibromyalgia After COVID
How common is fibromyalgia (widespread musculoskeletal pain) after COVID-19? Find out what a study showed.
Clinical Update
11/15/2021
Flu & COVID Vaccines
Additional research indicates that it's safe to get flu and COVID-19 vaccines together. Get the details.
Clinical Update
11/15/2021
Protection from Past COVID Infection?
Find out if past COVID-19 infection protects against re-infection 12 months later based on two recent studies.
Clinical Update
11/02/2021
Low-Dose Prednisone and COVID Vaccines
Find out if taking low-dose prednisone reduces immune response to the COVID-19 vaccine.
Clinical Update
9/28/2021
COVID + Flu Shot Timing
Find out if you can get a flu shot and COVID-19 shot at the same time and how this may affect side effects.
Clinical Update
9/28/2021
Flu Shot and Risk of COVID Complications
Getting a flu shot may actually reduce the risk of serious complications if you get COVID-19.
Clinical Update
Clinical Update
2/23/2022
Vaccination After COVID Infection
Getting vaccinated after COVID-19 appears to drastically reduce the risk of reinfection, according to a recent study.
Clinical Update
1/25/2022
Timing Vaccines Around COVID Drugs
Learn how long you need to delay vaccination after receiving drug therapy for COVID-19, including antivirals (such as remdesivir and Paxlovid) and antibody treatments.
Clinical Update
12/01/2023
COVID Vaccines: What to Know
Learn about the new COVID-19 vaccines including who should get them, who shouldn’t, and potential side effects.
Clinical Update
7/08/2022
Supplement for Post-COVID Fatigue?
Did supplementing with oxaloacetate reduce symptoms in people with post-COVID fatigue in a recent study? Find out in the Strategies for managing long COVID section of our article about How to Take Care of Yourself After You’ve Had COVID-19.
Clinical Update
2/25/2022
Zinc & COVID
Can taking zinc and vitamin C after being diagnosed with COVID-19 boost antibody production? Find out what a recent study found in the COVID section of our Zinc Supplements Review.
Also see our article about Supplements for COVID.
Clinical Update
9/21/2021
Medicine Helps Prevent COVID Symptoms
An FDA-authorized medicine was shown to reduce the risk of developing symptoms of COVID when given early to people testing positive for COVID. Get the details in our answer to the question: Do any treatments reduce the risk of infection after you've been exposed to someone with COVID-19?
Clinical Update
8/07/2021
COVID Transmission Among Vaccinated?
Can you catch COVID from someone who is vaccinated if you are vaccinated? Find out what data suggests in our article about COVID-19 Vaccines.
Clinical Update
8/07/2021
Vaccination in People Who've Already Had COVID
How much protection do vaccines provide to people who've already had COVID? See what a recent study suggests in our article about COVID-19 Vaccines.
Clinical Update
1/13/2021
Vitamin D & Risk of COVID Death
A recent study in the U.S. found that people hospitalized with COVID had much lower rates of death with certain blood levels of vitamin D. Get the details in the COVID-19 section of our Vitamin D Supplements Review. Also, see our Top Picks for vitamin D.
Clinical Update
3/01/2022
Check Your Area’s COVID Risk
You can now check if your area in the U.S. is at High, Medium, or Low risk for COVID and the CDC's mask recommendations for your area. Check your community's COVID-19 level.
Clinical Update
3/18/2022
Blood Type & Long COVID
People with a particular blood type appear more likely to experience long COVID, according to a recent study. Get the details in our answer to the question: How Should You Take Care of Yourself After You've Had COVID-19?
Clinical Update
7/01/2022
Don't Throw Out Expired COVID Tests
The shelf-lives of home COVID-19 tests have been extended by 3 to 7 months based on tests showing that many are still good past their printed expiration dates. Get the details for specific brands of tests in our article comparing COVID tests.
Clinical Update
8/05/2022
GABA for COVID?
Does taking GABA help with COVID? Find out what’s been shown in our updated answer to the question: Do any supplements help with COVID-19?
Clinical Update
12/13/2022
COVID Booster for Young Children
Children as young as 6 months may be able to get an updated COVID booster. Get the details about who is eligible for which vaccines in our CL Answer about COVID-19 vaccines.
Clinical Update
10/27/2023
Vitamin C & COVID
Did large, frequent doses of vitamin C help people hospitalized with COVID? Find out in the COVID-19 section of our Vitamin C Review. Also see our Top Picks for vitamin C.
Clinical Update
9/26/2023
Creatine for Post-COVID Fatigue?
Does supplementing with creatine reduce fatigue or other symptoms in people with post-COVID fatigue syndrome? Find out what a recent study showed in the Creatine - What It Does section of our Muscle & Workout Supplements Review. Also see our Top Picks for creatine.
Also see: How Should You Take Care of Yourself After You’ve Had COVID-19?
Clinical Update
1/02/2025
Fish Oil for Long COVID?
Does taking fish oil improve symptoms of long COVID such as shortness of breath, cough, fatigue, lack of taste, or loss of smell? Find out what a recent study showed in the COVID-19 section of our Fish Oil Supplements Review, which includes our Top Picks for fish oil.
Clinical Update
6/21/2022
COVID-19 Vaccination for Young Children
Find out how the newly authorized vaccinations for young children differ from each other in terms of dosing and number of doses.
Clinical Update
7/19/2022
Booster Benefit
Getting a second booster can significantly reduce the risk of COVID-19 hospitalizations among people 50 and older, according to a recent study.
Clinical Update
3/29/2022
Benefit of a Second Booster
Second boosters may significantly reduce the risk of severe COVID-19 in older people, according to a recent study.
Clinical Update
3/08/2022
More Home Tests Without Cost
Starting this week additional COVID-19 tests are available to U.S. residents without cost. Get the details.
Clinical Update
5/20/2022
At-Home Tests
You can now order 8 more COVID-19 antigen tests from the government. Get the details.
Clinical Update
4/29/2022
Vaccines and Appendicitis
Do COVID-19 vaccines increase the risk of appendicitis? Find out.
Clinical Update
2/01/2022
Vaccines, Blood Pressure, and Thyroid Symptoms
Find out if COVID-19 vaccines increase blood pressure or worsen symptoms of thyroid conditions.
Clinical Update
2/01/2022
Reinfection From Omicron
Be aware that if you had COVID-19 before Omicron, you’re a lot more likely to get reinfected now.
Clinical Update
8/03/2021
Ivermectin
Find out why ivermectin is generally not recommended for COVID-19 outside of clinical trials, despite some research suggesting a benefit.
Clinical Update
8/28/2021
J&J Booster Vaccine
Data suggests that a booster dose of the J&J vaccine can significantly boost immune protection against COVID-19. Get the details.
Clinical Update
8/25/2021
Ivermectin
More information highlights why ivermectin should be used for COVID-19 only within clinical trials. Get the details.
Clinical Update
8/17/2021
COVID Infectiousness
Be aware that even without symptoms, people with COVID-19 can emit high amounts of virus as aerosols, according to recent research. Get the details and find out what researchers recommend to help protect yourself when indoors.
Clinical Update
8/28/2021
Biotin Interference With COVID Tests
Be aware that taking biotin (vitamin B-7) may interfere with COVID-19 antibody tests. Learn which tests are affected. Also find out about other laboratory tests (including thyroid and cardiovascular tests) that can be affected by biotin.
Clinical Update
10/02/2021
Booster for Fully Vaccinated People Who've Had COVID?
Should fully vaccinated people who had COVID-19 get a booster? Find out.
Clinical Update
11/05/2021
Quercetin & COVID
Find out if quercetin helped people with early-stage COVID-19 in a recent study. Details are in the Viral Infections section of our Quercetin Supplements Review, which includes our test results and Top Picks among quercetin supplements.
Clinical Update
11/18/2021
Black Seed for COVID?
Can supplementing with black seed help improve symptoms or prevent severe disease among people with COVID-19? Find out what research suggests in our answer to the question: What are the health benefits of black cumin seed (black seed) oil?
Clinical Update
12/07/2021
Quercetin for Severe COVID?
Does quercetin help people with severe COVID-19? See what a recent study suggests in the Viral Infections section of our Quercetin Supplements Review. Also see our Top Picks for quercetin.
Clinical Update
1/28/2022
CBD To Reduce COVID Risk?
Use of a prescription formula was associated with lower risk of COVID-19 infection in a recent analysis, but there are important caveats to this finding. Get the details in the Colds, Flu, and Viral Infections section of our Review.
Clinical Update
5/13/2022
Paxlovid Interaction With Supplements
Paxlovid is strongly recommended for COVID treatment, but be aware that it may interact negatively with certain supplements, as well as many drugs. Get the details in CL Answer about COVID-19 treatments.
Clinical Update
6/07/2022
Caution Reading Rapid Tests
Many people incorrectly read rapid home tests for COVID-19, according to a recent study. Find out how to avoid the biggest mistake in our article about COVID tests.
Clinical Update
7/19/2022
COVID + Flu Vaccines
Getting a COVID-19 vaccine along with a flu shot seems to slightly increase the risk of side effects, according to a recent study. Get the details.
Clinical Update
8/05/2022
Do Eyeglasses prevent COVID?
Did wearing eyeglasses or sunglasses in public reduce the risk of getting COVID-19? Find out what a recent study showed in our CL Answer about face shields.
CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

News Release
February 24, 2022
Consumers Returned to Pre-Pandemic Supplement Usage in 2021, ConsumerLab Survey Reveals
White Plains, New York, February 24, 2022 —A survey of 8,049 people who use dietary supplements shows many supplements that declined in use in 2020 began bouncing back in 2021, such as magnesium (+2.4 percentage points), and CoQ10 (+2.7 pts).
Clinical Update
1/07/2022
Rapid, Home PCR Tests Compared
Should you use a home PCR test for COVID-19 instead of a home antigen test? Find out and learn which of three authorized home PCR tests is our Top Pick.
Clinical Update
1/07/2022
Testing After Isolation?
The CDC and American Medical Association have differing views on whether you should test negative before ending isolation due to COVID-19 infection.
Clinical Update
11/23/2021
COVID-19 Booster Shots: Who is Eligible?
Anyone over age 18 is now eligible for a booster six months after full mRNA vaccination or two months after the J&J vaccine, but find out which people are specifically encouraged to get a booster.
Clinical Update
11/26/2021
COVID-19 Booster Shots & Antibodies
How much antibody boost does a booster give? Much more than a second vaccine dose, a previous infection, or both, according to recent findings.
Clinical Update
11/26/2021
Fiberglass HEPA Filters
Are HEPA filters made out of fiberglass safe to use inside a home? Find out in our updated answer to the question: Which air purifiers are best for reducing the spread of COVID-19?
Clinical Update
10/02/2021
AstraZeneca Vaccine Efficacy & Side Effects
Find out how effective the AstraZeneca vaccine (not currently available in the U.S.) is at preventing COVID-19. Also learn the most common side effects of the AstraZeneca vaccine.
Clinical Update
1/21/2022
Supplement Interactions with Paxlovid
Certain supplements may interact with Paxlovid, the antiviral pill authorized for COVID-19 patients at high risk for severe disease. Find out which may interact.
Clinical Update
12/28/2021
Air Purifiers for Odor Removal?
Can air purifiers that remove virus particles from the air also eliminate odors? Find out in the Technologies for Removing Odors section of our answer to the question: Which air purifiers are best for reducing the spread of COVID-19?
Also see our Top Picks among air purifiers.
Clinical Update
8/31/2021
N95 Mask Quality Problem
Federal approval was recently revoked for all N95 masks made by a certain manufacturer. Get the details in our article about the Best Masks to Prevent COVID-19.
Clinical Update
3/31/2020
Is Quercetin Safe?
Quercetin has been proposed as a treatment for a range of medical conditions, including COVID-19, but is it safe? Find out what studies have found, including results of a recent, small clinical trial, in the Concerns and Cautions section of the Quercetin Supplements Review. Also see our Top Picks for quercetin.
Clinical Update
3/17/2020
How Long Does Coronavirus Last On Surfaces?
We've added new information about how long the coronavirus might last on packaging, and how this varies greatly depending on the package material, in our answer to the question: Can I get the new coronavirus (2019-nCoV) from supplements from China?
Clinical Update
4/14/2020
Zinc Concern
Zinc is a popular supplement right now due to COVID-19, but be aware that taking large doses of zinc for a long period of time can suppress the immune system by causing or exacerbating copper deficiency. Certain people may be more at risk for this. Get the details in the Cautions and Concerns section of the Zinc Supplements and Lozenges Review.
Clinical Update
5/20/2022
Pfizer Booster for Children
A Pfizer vaccine booster for children is now authorized by the FDA. Get the details in our updated article about COVID-19 Vaccines.
Clinical Update
5/24/2022
2nd Booster Recommendations
The CDC has strengthened its recommendation for the 2nd booster for people 50 and older and those 12 and older who are immunocompromised. Find out what prompted this change in our updated CL Answer about COVID-19 vaccines.
Clinical Update
6/01/2022
Disinfectants With Hypochlorous Acid
Are disinfectants with hypochlorous acid, such as Force of Nature and CleanSmart effective alternatives to harsher cleaning chemicals? Find out in our article about COVID-19 and Disinfecting.
Clinical Update
3/08/2022
Is 5-Day Isolation Enough?
More evidence suggests that isolating after a positive COVID-19 antigen test result for just 5 days may be too short.
Clinical Update
1/28/2022
Treatment Against Omicron
Find out which COVID-19 treatments (pills and infusions) are effective against Omicron and which are not.
Also learn how the length of hospital stays differs with Omicron versus Delta.
Clinical Update
2/04/2022
Shelf-Lives of Home COVID-19 Antigen Tests
If you’re storing a test for future use, be aware that shelf-lives range from just 5 ½ months to 12 months from time of manufacture. See how shelf-lives of popular brands compare.
Also see our Top Picks among antigen tests based on their accuracy and other features.
Clinical Update
2/08/2022
What To Do If You Test Negative, or Positive
We’ve summarized the latest information about when to use a home test and what to do if you get a negative, or a positive result. It’s all in the Antigen Tests section of our comprehensive article about testing for COVID-19.
Clinical Update
9/02/2022
Omicron-Targeting Boosters
The FDA has now authorized updated Pfizer and Moderna boosters. Find out who is eligible to get these shots and when, and learn who can still get the original boosters, in our updated CL Answer about COVID-19 vaccines.
Clinical Update
9/09/2022
Who Benefits Most from Paxlovid?
The efficacy of Paxlovid against severe COVID-19 infection may largely depend on the person's age, according to a recent study.
Clinical Update
8/09/2022
How Long Are You Contagious?
If you test positive on a rapid antigen test after a 5-day isolation, how likely are you to still be contagious? Find out in our CL Answer about COVID-19 testing.
Clinical Update
12/16/2022
Home PCR Test Recall
Certain lots of an over-the-counter PCR test have been recalled, affecting over 11,000 tests shipped to consumers. Get the details in our answer about COVID-19 tests.
CL Answer
What is TUDCA (tauroursodeoxycholic acid)? Does it have any proven health benefits, and is it safe?
TUDCA information, including its potential health benefits, safety, possible interactions, and cost.
Recalls & Warnings
February 02, 2023
DoTerra Sellers Ordered to Pay $15,000 for Deceptive COVID-19 Claims
On January 30, 2023, the website truthinadvertising.org reported that the U.S.
Clinical Update
9/14/2021
Vaccines Compared: Pfizer, Moderna, or J&J
See which vaccine (Pfizer, Moderna, or J&J) now appears to provide greatest protection against COVID-19-related hospitalization.
CL Answer
What are the health benefits of luteolin, and is it safe?
Find out if luteolin, a compound found in many fruits and vegetables, is beneficial for cancer, heart health, skin aging, age-related cognitive decline, and other conditions, and learn if it's safe.

CL Answer
Immuno 150: Does It Boost Immunity?
Immuno 150 by Exceptional Health Products is promoted for boosting the immune system, but does it work and is it worth its price? Find out.

Recalls & Warnings
February 01, 2022
Complaint Submitted to FTC for doTerra Essential Oil COVID Claims
On January 28, 2022, the website truthinadvertising.org submitted a complaint to the Federal Trade Commission (FTC) against doTerra International, LLC. following a series of Zoom calls from the company titled "Protocols for the Current Climate.
Recalls & Warnings
February 24, 2022
Seller of Essential Oils Warned for COVID-19 Claims
On February 22, 2022, the FDA issued a warning letter to My Natural Treatment following a review of the company’s website and Facebook page, which found statements about the company’s thyme, black seed, eucalyptus, rosemary, and peppermint oil products to be drug claims.
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
CL Answer
What is EpiCor? Does it really "boost" the immune system and prevent colds?
EpiCor supplement info, including evidence from clinical studies on immune function and cold/flu prevention, safety, and price.

Recalls & Warnings
May 08, 2023
SD Biosensor Pilot At-Home COVID-19 Tests Recalled Due to Bacterial Contamination
On May 4, 2023, the FDA warned consumers and health care providers not to use certain lots of Roche Diagnostics’ SD Biosensor, Inc. Pilot COVID-19 At-Home Tests due to bacterial contamination in the test kit’s liquid solution.
Recalls & Warnings
March 30, 2023
Seller of Tollovid Supplements Warned for COVID-19 Claims
On November 7, 2022, the FDA issued a warning letter to Todos Medical Ltd, aka Todos Medical USA Inc, following a review of the company’s websites, social media, and Amazon storefronts, which found statements about the company’s Tollovid 3CL Protease Inhibitor Delayed Release ...
Recalls & Warnings
January 12, 2023
FDA Warns Two Sellers Promoting CBD to Treat COVID-19
On January 10, 2023, the FDA issued warning letters to two companies following a review that found statements made on the companies’ websites to be drug claims because they promoted the cannabidiol (CBD) products to prevent or treat COVID-19.
Recalls & Warnings
December 01, 2022
FTC Takes Action Against Company Promoting “COVID Resist” Supplement to Treat COVID-19
On November 22, 2022, the FTC filed a complaint in a U.S. district court against California-based company Precision Patient Outcomes, Inc. for promoting its COVID Resist and VIRUS Resist supplements to prevent and treat COVID-19.
Recalls & Warnings
May 08, 2024
Razer, Inc. to Pay Over $1.1 Million Settlement for Zephyr Face Mask ”N-95” Claims
On April 29, 2024, the FTC announced it will be returning over $1.1 million to consumers who purchased Zephyr face masks after the company promoted its Zephyr face masks as N95-grade despite never submitting for testing to the FDA for approval of such claim.
Recalls & Warnings
November 03, 2022
Seller of CBD Warned for COVID-19 Claims
On November 1, 2022, the FDA issued a warning letter to Alternative Health Distribution LLC (d/b/a CannaAid) following a review of the company’s website, which found statements about the company’s cannabidiol (CBD) products to be drug claims.
Recalls & Warnings
January 10, 2023
Seller of CBD Warned for COVID-19 Claims
On January 5, 2023, the FDA issued a warning to PharmaCanna after review of the company’s website and social media, which found statements about the company’s cannabidiol (CBD) product, CBDefense 2000, to be drug claims because they promoted the products to prevent or treat ...
News Release
June 06, 2020
Best Vitamin C Supplements Revealed by ConsumerLab
White Plains, New York, June 6, 2020 — Due to its role in maintaining immune system health, vitamin C has been promoted to help prevent and treat COVID-19, as well as other viral infections such as colds.
News Release
April 28, 2020
Best and Worst Multivitamins Revealed by ConsumerLab -- Problems Found With 44% of Multis Tested
White Plains, New York, April 28, 2020 — Multivitamin/multimineral supplements are among the top five most popular supplements, but with so many different formulas on the market, it can be difficult to choose the right one.
Recalls & Warnings
March 09, 2023
"Dr. Rima Recommends" Nano Silver Recalled
On March 7, 2023, Natural Solutions Foundation issued a recall of Dr. Rima Recommends Nano Silver 10ppm Dietary Supplement, which was promoted to prevent and/or treat COVID-19.
Recalls & Warnings
October 30, 2020
Seller of Vitamin C, Vitamin D, Ashwagandha, and More Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Predator Nutrition for selling the products Elixir, Salidroside, Unbreakable, Vitamin C + Bioflavonoids & Rosehip, Vitamin D3, and Ashwagandha with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Seller of Elderberry and Honey Products Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Beepothecary LLC for selling the products BEEHive Delight, BEEbread, and Elderberry, Honey & Propolis Syrup with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Simple Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 23, 2020, the FDA issued a warning letter to Peterson Research Laboratories LLC for selling the product Simple Silver with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
April 27, 2022
FDA Warns Manufacturer of Topical Antiseptic Products for COVID Claims
On April 19th, 2022, the FDA issued a warning letter to Kleenhanz, LLC following a review of the company’s website and social media which found statements about the company’s Kleenhanz Towelettes topical antiseptic products to be drug claims.
Recalls & Warnings
December 01, 2020
Niagen Cannot Be Promoted to Prevent or Treat COVID-19, Warns FDA
On November 17, 2020, the FDA issued warning letters to the sellers of Niagen and Nadovim, supplements that contain different forms of niacin, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 11, 2020
Seller of "Dr. Hotze's Immune Pak" Products Warned for COVID-19 Claims
Seller of "Dr. Hotze's Immune Pak" Products Warned for Coronavirus Claims
Recalls & Warnings
March 09, 2022
FDA Warns Seller of Magnesium, CBD, Herbal Extracts & More
On February 9, 2022, the FDA issued a warning letter to Bea Lydecker’s Naturals, Inc.
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Liposomal Vitamin C, Vitamin D & More
On December 21, 2020, the FDA issued a warning letter to Sparrow Health & Performance LLC for selling the products Organic Liposomal Vitamin C, Nanoemulsified D3K2 (also called Liquid Liposomal Vitamin D3 with K2 or Liposomal Vitamin D3) and Immune Support ...
Recalls & Warnings
April 02, 2021
FDA Warns Sellers of Prostate, Reishi, Immune Products, and More
On March 16, 2021, the FDA issued warning letters to two companies following reviews of their websites which found statements made about the companies' products to be drug claims.
Recalls & Warnings
March 16, 2021
FDA Warns Seller of Essential Oils
On March 12, 2021, the FDA issued a warning letter to Ravenscroft Apothecary, Inc.
Recalls & Warnings
March 09, 2021
CBD Seller Warned for Drug and COVID-19 Claims
On March 1, 2021, the FDA issued a warning letter to Cannafyl following a review of the company's website, which found statements made about the company's products Balance CBD Drops, Relief CBD Drops, Relax CBD Drops and Relief CBD Salve to be drug claims.
Recalls & Warnings
January 25, 2021
Washington Resident Arrested for Selling Illegal COVID-19 Vaccine
On January 21, 2021, 55-year-old Washington resident Johnny T. Stine was arrested for selling and administering illegal, home-made COVID-19 vaccines to Americans.
Recalls & Warnings
April 23, 2021
FDA Warns 5 Sellers of Unapproved COVID-19 Tests
Between March 18 and April 6, 2021, the FDA issued warning letters to five companies for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Flu Immune
On December 21, 2020, the FDA issued a warning letter to Riverstone LLC for selling the products Flu Immune Drops, L-Lysine, Lysine Extra, and Monolaurin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 11, 2020
Colloidal Silver Seller Warned for COVID-19 Claims
On December 2, 2020, the FDA issued a warning letter to Heavenly Natural Products for selling various Carbon 60 and colloidal silver products, including AVOCADO C60 ANTI-VIRAL COMBO — VIRUS PREVENTION (made with C60 WITH AVOCADO and Heavenly Silver) with unsupported ...
Recalls & Warnings
November 02, 2020
Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 30, 2020, the FDA issued a warning letter to Spartan Enterprises Inc. dba Watershed Wellness Center for selling the silver product Dissolve BioActive Silicate with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
March 05, 2021
Seller of Earth Tea Warned for COVID-19 Claims
On February 18, 2021, the FDA issued a warning letter to B4B Corp. following a review of the company's website by the FDA and Federal Trade Commission (FTC) which found the company promoted Earth Tea Extra Strength with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
February 17, 2021
FDA Warns Dr. Paul's Lab for COVID-19 Claims
On February 16, 2021, the FDA issued a warning letter to Dr. Paul's Lab following a review of the company's website by the FDA and Federal Trade Commission (FTC) for selling COVID-Aid Tincture with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
February 16, 2021
Seller of BioCBD+ Products Warned for COVID-19 and Drug Claims
On February 11, 2021, the FDA issued a warning letter to Evolved Ayurvedic Discoveries, Inc.
Recalls & Warnings
March 02, 2021
FDA Warns Seller of Vitamin C, Silver Spray
On March 1, 2021, the FDA issued a warning letter to Ageless Global, LLC following a review of the company's websites for selling Immunoral, Immune Plus, MD Immune Support Spray, and MD CVK-365 Mouth Spray with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 08, 2021
FDA Warns Seller of "CoronaBox" Containing Vitamin D, Probiotics & More for Unsupported Claims
On May 24, 2021, the FDA issued a warning letter to Everything Health LLC following a review of the company's website by the FDA and Federal Trade Commission (FTC) which found the company promoted its CoronaBox (which contains cordyceps, vitamins, and K2, magnesium, ginger, probiotics ...
Recalls & Warnings
January 20, 2021
FDA Warns Seller of Vitamin D, Curcumin and CoQ10 Promoted for Treating COVID-19
On December 28, 2020, the FDA issued a warning letter to Smarter Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Smarter Curcumin, Smarter Ubiquinol, and Smarter Vitamin D3 to be drug claims.
Recalls & Warnings
January 12, 2022
FDA Warns Consumers Not to Use LuSys Laboratories COVID Tests Due to False Results
Update: (03/09/22) On February 17, 2022, the FDA issued a warning letter to LuSys Laboratories, Inc.
Recalls & Warnings
April 20, 2021
Chiropractor Who Claimed Vitamin D and Zinc Work Better Than COVID Vaccines Charged by FTC
On April 15, 2021, the FTC charged St.
Recalls & Warnings
July 14, 2022
FDA Warns Seller of Unauthorized COVID-19 Tests
On June 30, 2022, the FDA issued a warning letter to W.H.P.M, Inc. for distributing COVID antigen tests without approval, clearance, or authorization from the FDA while claiming to mitigate, prevent, treat, diagnose, or cure COVID-19 in people.
Recalls & Warnings
August 08, 2022
Woman Pleads Guilty for Selling and Promoting Products to Treat COVID-19
On July 27, 2022, Diana Daffin, owner of Savvy Holistic Health pleaded guilty to selling products with claims they could treat COVID-19 and promised “Immunity for Humans.” The products were sold under the HAMPL brand name.
Recalls & Warnings
August 08, 2022
Seller of CBD Warned for COVID-19 Claims
On August 4, 2022, the FDA sent a warning letter to FluxxLab LLC following a review of the company’s website and social media which found statements about the company’s Covid-19 Immune Support Tincture and CBDA+CBD Oil Tincture products to be drug claims because they ...
Recalls & Warnings
December 22, 2021
Unauthorized Rapid Antigen Test Gets FDA Warning
On December 1, 2021, the FDA issued a warning letter for DermaCare Biosciences, LTD.
Recalls & Warnings
October 11, 2023
Family Sentenced to Over 12 Years in Prison for Selling Dangerous “Bleach” Miracle Mineral Solution as “COVID Cure”
Four Florida men who distributed the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions have been sentenced to 5 to 12 years in prison for conspiring to defraud the United States.
Recalls & Warnings
December 02, 2020
Seller of Vitamin C, Elderberry, Silver, and More Warned for COVID-19 Claims
On November 10, 2020, the FDA issued a warning letter to Sage Woman Herbs, Ltd.
Recalls & Warnings
December 11, 2020
FDA Warns iThrive.health for Promoting Supplements as COVID-19 Treatments
On December 10, 2020, the FDA issued a warning letter to iThrive.health for promoting the sale of various vitamins and supplements on Amazon.com with claims that they can mitigate, prevent, treat, diagnose, or cure COVID-19.
Recalls & Warnings
December 29, 2021
Natural Solutions Foundation Banned From Selling "Nano Silver" as COVID Treatment
On December 28, 2021, the United States Department of Justice announced that Natural Solutions Foundation has agreed to stop selling its nano silver products, in order to settle a suit brought against the company by the FDA for claiming the product could that the company claimed could prevent, ...
Recalls & Warnings
March 04, 2022
FTC, FDA, and DOJ Take Joint Action Against Herbal Tea Companies for COVID Claims
On March 3, 2022, the FDA, FTC, and DOJ sued a New York-based marketer of herbal tea, in attempts to permanently block deceptive ads that claim Earth Tea is clinically proven to treat, cure, and prevent COVID-19.
Recalls & Warnings
May 09, 2022
$149 Million in Refunds from AdvoCare Settlement
On May 5, 2022, the FTC announced it will be returning more than $149 million to individuals who sold AdvoCare supplements as distributors through the company’s multi-level-marketing (MLM) plan.
Recalls & Warnings
January 31, 2023
Over $973,000 Returned to NutraClick Consumers
On January 25, 2023, the FTC announced it will be returning over $973,000 to over 17,000 consumers who lost money after NutraClick LLC allegedly automatically enrolled them in unwanted membership programs for supplements and beauty products.
Recalls & Warnings
January 21, 2021
Federal Court Bars Fusion Health From Promoting Vitamin D for COVID-19
On January 8, 2021, the United States Department of Justice announced a permanent injunction has been entered, barring dietary supplement marketer Matthew Ryncarz and his companies Fusion Health and Vitality LLC dba Pharm Origins and Fusion Ionz LLC dba Pharm Origins from making claims that their ...
Recalls & Warnings
May 28, 2020
FDA Warns Sellers of CBD, Colloidal Silver, Essential Oils, and More Promoted to Treat Coronavirus
On May 26, 2020, the FDA issued warning letters to four companies for selling products such as CBD, essential oils, colloidal silver, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 29, 2020
FDA Warns Consumers about Fraudulent Tests, Vaccines, and Treatments for COVID-19
On September 21, 2020, the FDA released a statement warning consumers not to buy or use "questionable products" that claim to help diagnose, treat, cure, or prevent coronavirus (COVID-19).
Recalls & Warnings
June 23, 2020
North Isle Wellness Center Warned for Coronavirus Claims
On June 19, 2020, the FDA issued a warning letter to North Isle Wellness Center for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 19, 2020
FDA Warns Sellers of CBD, Kratom, Vitamin C, and More for Coronavirus Claims
Between May 11 and May 14, 2020, the FDA issued warning letters to five companies for selling products such as vitamin C, immune boosters, kratom, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 12, 2020
FDA Warns Seller of "COVID-19 Cough Syrup"
On May 8, 2020, the FDA issued a warning letter to Seanjari Preeti Womb Healing, L.L.C. for selling its product COVID-19 Cough Syrup with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
May 12, 2020
Plum Dragon Herbs Warned for Coronavirus Claims
On May 8, 2020, the FDA issued a warning letter to Plum Dragon Herbs, Inc. for selling Chinese medicine products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 17, 2020
The Art of the Cure Warned for Coronavirus Claims
On April 15, 2020, the FDA issued a warning letter to The Art of the Cure for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 07, 2020
Sellers of Essential Oils, Hand Sanitizers and More Warned for Coronavirus Claims
The FDA recently issued warning letters to five companies for selling products such as essential oils, homeopathic products, and Chinese herbal products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 03, 2020
Seller of "COVID Supplement Protection Pack" Warned for Coronavirus Claims
On June 30, 2020, the FDA issued a warning letter to Center for Wellness and Integrative Medicine for selling the products COVID Supplement Protection Pack (also referred to as the COVID Household Value Pack), Thymosin-Alpha, and Methylene Blue Capsules with unsupported ...
Recalls & Warnings
October 12, 2020
Griffo Botanicals Warned for COVID-19 Claims
On October 7, 2020, the FDA issued a warning letter to Griffo Botanicals for selling various herbal products, including BaseCamp, Gan Mao, Febris, Fortifend, Xiao Chai Hu, HuBei #1, Qing Fei Pai Du, Solis, and WuHan #2, with unsupported claims that they can treat coronavirus ...
Recalls & Warnings
October 02, 2020
Tonic Therapeutic Herb Shop & Elixir Bar Warned for COVID-19 Claims
On September 29, 2020, the FDA issued a warning letter to Tonic Therapeutic Herb Shop & Elixir Bar for selling various herbal products, including Tonic Therapeutic Herb Shop & Elixir Bar, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 18, 2020
Colloidal Silver Seller Warned for Coronavirus Claims
On August 14, 2020, the FDA issued a warning letter to SilveryGuy for selling colloidal silver products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 13, 2020
FDA Warns Three More Companies Selling Unapproved COVID-19 Tests
Between July 23 and 24, the FDA issued warning letters to three companies for marketing unapproved, adulterated or misbranded antibody tests for coronavirus (COVID-19).
Recalls & Warnings
July 17, 2020
Sellers of Immune Boosters, Vitamin C Warned for Coronavirus Claims
The FDA recently issued warning letters to two companies for selling products such as immune boosters, vitamin C, and zinc with unsupported claims that they can treat COVID-19 (use the links below to read the full warning letter):
Recalls & Warnings
August 13, 2020
FDA Warns Seller of Chaga for Coronavirus Claims
On August 6, 2020, the FDA issued a warning letter to Canadian Chaga for selling 124 Chaga Capsules, Chaga Tea, and Canadian Chaga Tincture with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 15, 2020
FDA Warns Seller of Unapproved "Wondfo Novel Coronavirus Antibody Detection Kit"
On June 29, 2020, the FDA issued a warning letter to Epro E-Commerce Limited dba DealExtreme and DX.com for selling Wondfo Novel Coronavirus (2019-nCoV) Antibody Detection Kit, an unapproved, adulterated, and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
May 02, 2020
Hopewell Essential Oils Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Hopewell Essential Oils for selling its essential oils with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 03, 2020
FDA Warns Pure Plant Essentials for Essential Oil Coronavirus Claims
On April 1, 2020, the FDA issued a warning letter to Health Mastery Systems DBA Pure Plant Essentials for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 03, 2020
FDA Warns Seller of Homeopathic Medicine for Coronavirus Claims
On April 1, 2020, the FDA issued a warning letter to Homeomart Indibuy for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 11, 2020
Seller of "COVID PACK" Warned for Coronavirus Claims
On September 9, 2020, the FDA issued a warning letter to Pharmacy Plus, Inc. dba Vital Care Compounder for selling COVID PACK and COVID 'POSITIVE' PACK products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 12, 2020
Prairie Dawn Herbs Warned for COVID-19 Claims
On October 7, 2020, the FDA issued a warning letter to Prairie Dawn Herbs for selling various herbal products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 19, 2020
Seller of Thrive "Anti-Viral" Supplements Settles with FTC for Unproven Coronavirus and Cancer Claims
On October 19, 2020, the Federal Trade Commission (FTC) approved an administrative consent order to settle charges that marketer Marc Ching was making unsubstantiated claims that the supplement Thrive can treat, prevent, or reduce the risk of coronavirus (COVID-19).
Recalls & Warnings
June 29, 2020
Seller of Chinese Herbal Medicines Warned for Coronavirus Claims
On June 26, 2020, the FDA issued a warning letter to SuperHealthGuard and Loyal Great International Ltd. for selling traditional Chinese medicine products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 23, 2020
KBMO Diagnostics Warned for Unapproved COVID-19 Test
On June 17, the FDA issued a warning letter to KBMO Diagnostics, LLC for selling the product COVID-19 Fingerstick Test Kit, an unapproved, adulterated and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
May 02, 2020
Seller of Botanical and CBD Oil Patches Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Santiste Labs LLC for promoting its transdermal patches containing botanical oils and/or CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 20, 2020
FDA Warns Companies Selling Unapproved COVID-19 Tests
On June 17, the FDA issued warning letters to three companies for marketing unapproved, adulterated or misbranded antibody tests for coronavirus (COVID-19).
Recalls & Warnings
June 05, 2020
Seller of Silver and Vitamin C Lozenges & More Warned for Coronavirus Claims
On June 1, 2020, the FDA issued a warning letter to Dr.
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 23, 2020
Seller of CBD Tinctures and "Immune Boost Packs" Warned for Coronavirus Claims
On June 18, 2020, the FDA issued a warning letter to Project 1600 Inc. for selling CBD products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 15, 2020
FDA Warns Seller of Unapproved "COVID-19 test package"
On June 29, 2020, the FDA issued a warning letter to Pomegranate Consulting, LLC, Pomegranate Consulting, Ltd. dba Glorious One-Pot Meals for selling COVID-19 test package, an unapproved, adulterated, and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
August 25, 2020
Seller of CBD and NAC Warned for Coronavirus Claims
On August 19, 2020, the FDA issued a warning letter to Living Senior, LLC for selling CBD and NAC products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 10, 2020
Seller of Hand Sanitizer "Alternative" Warned for Coronavirus Claims
On July 7, 2020, the FDA issued a warning letter to Ionogen, LLC for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 14, 2020
FTC Warns Companies Selling Immune "Boosters," Vitamin C and More for Coronavirus Claims
On April 14, 2020, the FTC announced that it has sent warning letters to ten companies for selling products such as immune boosters, silicone facial brushes, air purifiers, and intravenous vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 17, 2020
Earth Angel Oils Warned for Coronavirus Claims
On April 14, 2020, the FDA issued a warning letter to Earth Angel Oils for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 10, 2020
Seller Indicted for Promoting Silver Product as Coronavirus Cure
On July 28, 2020, Utah resident Gordon Pedersen was indicted by a federal grand jury for posing as a medical doctor to sell an unapproved treatment for coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
Federal Court Orders Seller to Stop Promoting Silver Product as Coronavirus Cure
On April 29, 2020, a federal court in Utah announced that it has obtained a temporary restraining order preventing Gordon Pedersen and his companies, My Doctor Suggests LLC and GP Silver LLC, from promoting fake treatments for coronavirus (COVID-19).
Recalls & Warnings
May 26, 2020
Seller of Vitamin and CBD "NoronaPak" Warned for Coronavirus Claims
On May 21, 2020, the FDA issued a warning letter to Apollo Holding LLC for promoting a kit containing vitamins and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 03, 2020
FDA Warns Seller of FullerLifeC60 for Coronavirus Claims
On March 30, 2020, the FDA issued a warning letter to FullerLifeC60, LLC, for selling a product with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
April 11, 2020
FDA Warns Sellers of CBD, Colloidal Silver & Natural Remedies Promoted to Treat Coronavirus
Between April 7 and April 9, 2020, the FDA issued warning letters to five companies for selling products such as CBD, colloidal silver, and natural treatments with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 09, 2020
Six More Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On June 5, 2020, the FTC announced that it sent warning letters to six multi-level marketing companies for selling products such as immune system boosters and probiotics with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrepresenting potential earnings people who have ...
Recalls & Warnings
March 09, 2020
FDA Warns Sellers of Essential Oils, Colloidal Silver & Teas Promoted to Treat Coronavirus
On March 9, 2020, the FDA and FTC announced they have issued joint warning letters to seven companies for selling products such as essential oils, teas and colloidal silver with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 11, 2020
FDA Warns Seller of Products Promoted to Treat Coronavirus in Pets and Humans
On April 7, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet for selling products intended for pets and humans with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 10, 2020
Seller of Thrive "Anti-Viral" Supplement Barred from Making Unproven Coronavirus and Cancer Claims
On July 10, 2020, the Federal Trade Commission (FTC) barred marketer Marc Ching from making unsubstantiated claims that the supplement Thrive can treat, prevent, or reduce the risk of coronavirus (COVID-19). Thrive consists mainly of vitamin C and herbal extracts.
Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Clinical Update
10/21/2022
Who's Most Likely to Get Severe COVID?
Among people who were vaccinated but later got COVID, a new analysis shows who’s mostly likely to get severe COVID depending on vaccine brand, age, medications taken, and health conditions. See the details in our article about COVID vaccines.
Clinical Update
9/07/2021
COVID Symptoms in Breakthrough Cases
Fully vaccinated people who get COVID are much less likely to experience symptoms, as well as long-COVID, than unvaccinated people with COVID to according to a recent study. Get the details.
Clinical Update
11/11/2021
Long COVID Symptoms Not Due to COVID?
A recent study suggests that "long COVID" symptoms reported by some people may not be due to COVID. Find out why this is of concern.
Recalls & Warnings
April 21, 2020
CanaBD Warned for CBD Coronavirus Claims
On April 16, 2020, the FDA issued a warning letter to Nova Botanix LTD DBA CanaBD for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 18, 2020
Seller of Immune Shot Criminally Charged With Making Coronavirus Claims
On August 10, 2020, prosecutors in Georgia charged Matthew Ryncarz and his company Fusion Health and Vitality, LLC d/b/a/ Pharm Origins with selling the misbranded product Immune Shot.
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 03, 2020
Gaia's Whole Healing Essentials Warned for Colloidal Silver Coronavirus Claims
On April 1, 2020, the FDA issued a warning letter to Gaia's Whole Healing Essentials, LLC, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 01, 2020
NeuroXPF CBD Warned for Making Coronavirus Claims
On March 31, 2020, the FDA issued a warning letter to NeuroXPF for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 14, 2020
Herbs of Kedem Warned for Making Coronavirus Claims
On April 10, 2020, the FDA issued a warning letter to Herbs of Kedem for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 31, 2020
FTC Sues Golden Sunrise for Marketing Deceptive $23,000 Coronavirus Cure
On July 31, 2020, the Federal Trade Commission (FTC) charged Golden Sunrise Nutraceutical, Inc., a California-based company, with deceptively advertising a $23,000 treatment plan as a scientifically proven way to treat COVID-19.
Recalls & Warnings
March 29, 2020
FDA Warns Seller of Nasal Spray Promoted for Coronavirus
On March 26, 2020, the FDA issued a warning letter to Corona-cure.com for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 21, 2020
U.S. Attorney's Office Bars Chiropractor from Selling Fake Coronavirus Cures
On April 17, 2020, the United States Attorney's Office for the Northern District of Texas announced that it has obtained a temporary restraining order preventing a chiropractor from promoting fake treatments for coronavirus (COVID-19). Dr. Ray L.
Recalls & Warnings
March 13, 2020
State of Missouri Sues Jim Bakker Show for Selling False Coronavirus Cure
On March 12, 2020, the Missouri Attourney General announced that it sued the Jim Bakker Show for selling a fake coronavirus (COVID-19) cure called Silver Solution.
Recalls & Warnings
March 29, 2020
Seller of "Immune Tonic" Warned for Making Coronavirus Claims
On March 26, 2020, the FDA issued a warning letter to Carahealth for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 28, 2020
Ten Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On April 24, 2020, the FTC announced that it sent warning letters to ten multi-level marketing companies for selling products such as essential oils and immune system boosters with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrespresenting potential earnings people ...
Recalls & Warnings
May 09, 2020
FTC Warns 45 More Companies for Coronavirus Claims
On May 7, 2020, the FTC announced that it sent warning letters to 45 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Clinical Update
11/09/2021
COVID Booster & Risk of Spreading Infection
A booster reduces the amount of virus you produce if you get COVID, according to a recent study. This should help reduce the risk of spreading infection if you get COVID.
Clinical Update
11/09/2021
Antibody Levels: Having Had COVID vs. Being Vaccinated
How does having had COVID compare to being vaccinated in terms of antibody levels? See what a recent study found, as well as the effect of vaccination after having had COVID.
Clinical Update
9/28/2021
Risk of Infecting Others
While vaccines are good at keeping you from getting severely ill from COVID, be aware that if you get COVID, you may be as infectious as a non-vaccinated COVID patient, according to several recent reports.
Clinical Update
11/18/2021
Fish Oil for COVID?
Does taking concentrated fish oil reduce hospitalizations or deaths in people with COVID? Find out what a study showed in the COVID section of our Fish and Marine Oil Supplements Review.
Clinical Update
12/07/2021
Chances of COVID Reinfection with Omicron Variant
If you've had COVID, be aware that the chance of getting COVID a second time is much higher with the Omicron variant than with previous variants, according to recent data.
Clinical Update
9/11/2021
Flu Vaccine & COVID
Learn if you need to time your flu vaccine around your COVID vaccine and how the flu vaccine may affect you if you later get COVID. Also find out when kids should get a flu shot.
Clinical Update
9/24/2021
Resveratrol for Mild COVID?
A recent study gave resveratrol to people with mild COVID. Find out if it reduced hospitalization, ER visits or pneumonia in the What It Does section of our Resveratrol Supplements Review.
See information about the use of other supplements for COVID.
Clinical Update
2/11/2022
Vitamin D & COVID
What vitamin D levels are associated with the lowest risk of getting severe COVID?
Does raising vitamin D levels shorten the duration of COVID symptoms? See what a recent study found.
Also see our Top Picks for vitamin D.
Clinical Update
8/30/2022
Four More Days to Get COVID Tests
If you haven't already ordered yours, you have only until September 2 to receive at-home COVID tests from the U.S. government without cost. These may be particularly useful if cases rise in the fall and winter. Get the details in our article about Finding the Best COVID Tests.
Clinical Update
9/26/2023
COVID Tests
Each U.S. household can now order a set of four at-home COVID tests from the government. Get the details in our article about the best COVID tests.
Clinical Update
12/27/2023
Best At-home COVID Tests -- Updated
Find out which at-home COVID tests are most accurate, easy to use, and currently available in our updated article, which includes performance comparisons and our Top Picks among at-home COVID tests.
Clinical Update
Recalls & Warnings
June 25, 2020
"Anti-Viral" Supplement Recalled for Unsupported Coronavirus Claims
On June 23, 2020, Golden Nutrition Inc. issued a recall of four lots of Anti-Viral Immune Enhancement Capsules because the label makes unsupported health claims to "help fight corona virus and influenza.
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Clinical Update
11/15/2021
COVID Vaccines & Shingles
Do COVID vaccines activate shingles? Find out what research suggests.
Clinical Update
10/08/2021
Home COVID Test Recalled
Certain lots of a popular home COVID test have been recalled after they were discovered to provide inaccurate results.
Clinical Update
10/09/2021
Substance Abuse & COVID
People with substance use disorders, particularly regarding cannabis, may be at increased risk for COVID infection, according to a recent study.
Clinical Update
Clinical Update
12/24/2021
At-Home COVID Testing
Best time to take an at-home COVID test? It may be sooner than you think.
Clinical Update
1/14/2022
At-Home COVID Tests Without Charge
Find out where to get COVID tests without charge in your area and how to get reimbursed for buying at-home antigen tests (which starts tomorrow).
Clinical Update
9/03/2021
Kidney Injury and COVID
Kidney injury appears to be more common in some people who have had COVID, according to a recent study.
Clinical Update
8/31/2021
Adolescents & COVID: Myocarditis vs. Hospitalization
How does the risk of myocarditis from vaccination compare to the risk of hospitalization from COVID among adolescents?
Clinical Update
8/31/2021
Off-Label Use of COVID Vaccine?
Find out why vaccine administrators may decline to give COVID shots for off-label use.
Clinical Update
8/14/2021
COVID Vaccination During Pregnancy
Vaccination is now recommended during pregnancy. Get the latest details in our article about COVID Vaccines.
Clinical Update
8/28/2021
15-Minute Home COVID Tests
Demand for 15-minute home tests for COVID is increasing. Learn when and how to use these tests.
Clinical Update
Recalls & Warnings
June 20, 2020
FTC Warns 30 More Companies for Coronavirus Claims
On June 18, 2020, the FTC announced that it sent warning letters to 30 companies for selling products such as immune system boosters, colloidal silver, vitamin C, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 02, 2020
FDA Warns Sellers of Unapproved COVID-19 Tests, CBD Products
On November 23 and November 30, 2020, the FDA issued warning letters to Industry Lab Diagnostic Partners and Avazo-Healthcare, LLC, respectively, for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
Recalls & Warnings
April 26, 2021
Family Indicted for Selling Dangerous "Bleach" Miracle Mineral Solution As "COVID" Cure
Four Florida men have been indicted by a Miami federal grand jury on charges of fraudulently marketing and selling the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions, such as cancer, Alzheimer's, diabetes, autism, malaria, ...
Clinical Update
10/02/2024
Low-dose Lithium for Long COVID?
Did taking low-dose lithium improve symptoms of long COVID? Find out what a recent study showed in the What It Does section of our Low-Dose Lithium Review, which includes our Top Picks for low-dose lithium.
Clinical Update
5/05/2023
May 11 Deadline for Tests
Until May 11, U.S., households are eligible to receive four at-home COVID tests from the government if not already ordered this year. Get the details in our article about COVID Tests, which includes our Top Picks among at-home rapid antigen tests.
Clinical Update
12/02/2022
Don’t Throw Out Expired COVID Tests
The shelf-life for some at-home COVID antigen tests was recently extended by several months - so don't throw them away! Get the details by brand.
Clinical Update
Clinical Update
9/02/2022
CoQ10 for Post-COVID Fatigue?
Can CoQ10 supplementation reduce physical and mental fatigue in people experiencing prolonged COVID symptoms? See what a recent study found in the What It Does section of our CoQ10 Supplements Review.
Clinical Update
8/28/2021
Six or Eight Months for mRNA COVID Booster?
Will mRNA booster vaccines be given as early as 6 months after the first dose, or at 8 months as had been officially announced? Find out in our article about COVID Vaccines.
Clinical Update
8/22/2021
COVID Vaccine 8-Month Booster
Learn about the waning efficacy of COVID vaccines, why the booster is being offered, why at 8 months, as well as who can get it, when and how.
Clinical Update
8/31/2021
Risk of Hospitalization With COVID
If you are unvaccinated, how much more likely are you to be hospitalized with COVID than if you were vaccinated? See what recent data shows.
Clinical Update
8/31/2021
Delta Variant Accounts for Most COVID Cases
Be aware that the Delta variant, which is more transmissible than other variants, now accounts for more than 98% of COVID cases in the U.S. See the latest numbers.
Clinical Update
9/24/2021
Antibody Therapy for COVID
Due to a shortage in supply, there has been a change in how antibody therapies for COVID are being distributed in the U.S. Find out how this may affect your ability to access these therapies.
Clinical Update
12/16/2020
High-Dose Fish Oil for COVID?
A two-week course of high-dose, high-concentration fish oil was reported to reduce inflammation and symptoms in COVID patients in a preliminary study. See the details in the What It Does section of the Fish Oil Supplements Review. Also, see our Top Picks for fish oil supplements.
Clinical Update
12/10/2021
Home COVID Tests
Details are still being worked out, but see the latest information about how the U.S. government intends to make COVID self-tests freely available or reimbursable depending whether you are insured, uninsured, or covered by Medicaid or Medicare. Also see our Top Pick among 7 brands of home tests.
Clinical Update
12/03/2021
Best Rapid Home Test for COVID
The FDA has authorized many 15-minute home tests for COVID, but some are better than others at identifying positive samples. See our Top Pick among rapid home tests and how brands compare.
Be aware that these home tests, which can be bought without a prescription, are best used within six days of the start of symptoms. It is likely that they can detect the new Omicron variant. The cost of buying these tests may soon be covered by insurance.
Clinical Update
12/03/2021
Home Pulse Oximeter Use Decreases Risk of COVID Death
Using a pulse oximeter at home to monitor blood oxygen levels was associated with a significant decrease in risk of death among people with COVID, according to a new study. Get the details and see our Top Pick among pulse oximeters.
Clinical Update
12/07/2021
More COVID Home Tests Reviewed — See If They Made Our Cut
The FDA recently authorized many more over-the-counter rapid (antigen) home tests for COVID, but there are significant differences in their accuracy.See how 7 widely sold brands compare, where we've set the bar between the best and the rest, and our Top Pick. Just yesterday, we added information about four more tests.
Clinical Update
10/12/2021
More About Shingles Vaccines & COVID
We recently reported that getting a shingles vaccine was linked to lower COVID risk. A CL member asked us if this association applied to Shingrix, Zostavax or both vaccines. See the answer.
Clinical Update
11/05/2021
Flu Shot + COVID Booster?
Find out if it's okay to get a flu shot at the same time as a COVID booster based on recent evidence.
Clinical Update
10/08/2021
Home Device May Reduce Risk of COVID Death
New evidence suggests that using a pulse oximeter when home with COVID may reduce the risk of death. We've added information about this to our answer to the question: Which is the best pulse oximeter for home use?
Clinical Update
3/31/2022
How to Get COVID Treatment Quickly
Antivirals work against COVID if given early. Some pharmacies can now give you antiviral medication without a prescription from your doctor if you test positive, saving valuable time. Learn how and find the location nearest you.
Clinical Update
3/25/2022
No-Cost COVID Vaccine Eligibility Ending?
Take note: After April 4, some people in the U.S. may no longer be able to get a COVID vaccine without paying out of pocket.
Clinical Update
3/04/2022
Caution: Many Still Positive After 5 Day Isolation
People with COVID must isolate for 5 days, but many are positive on antigen tests for up to 9 days. Find out who is most at-risk for this, in our updated answer about COVID Tests.
Clinical Update
2/15/2022
New COVID Treatment
An antibody treatment that works against Omicron infection was recently authorized for people at high risk of progression to severe disease, but it needs to be used early. Learn more about it and other treatments in our answer about COVID treatments.
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 10, 2020
FTC Charges Seller With Falsely Promising Consumers Next Day Shipping of Face Masks and Other PPE
On July 8, 2020, the Federal Trade Commission (FTC) charged the online marketer SuperGoodDeals.com, Inc. and its owner, Kevin J. Lipsitz, with falsely promising consumers next-day shipping of facemasks and other personal protective equipment (PPE) to use during the coronavirus pandemic.
Recalls & Warnings
July 14, 2020
Sellers of Miracle Mineral Solution Criminally Charged With Making Coronavirus Claims
On July 8, 2020, federal prosecutors charged the sellers of Miracle Mineral Solution, a toxic bleach solution marketed as a cure for coronavirus (COVID-19), with conspiracy to defraud the United States, conspiracy to violate the Federal Food, Drug and Cosmetic Act, and criminal contempt.
Recalls & Warnings
July 06, 2021
CBD Products Were Promoted to Treat Cancer and Alzheimer's Without Proof, Says FTC
On July 6, 2021, the Federal Trade Commission (FTC) announced that it has approved a final administrative consent orders against Kushly Industries LLC and the company's owner, Cody Alt, for allegedly making unsupported health claims about its CBD products.
Recalls & Warnings
May 04, 2023
FDA Warns MedoLife for Promoting Homeopathic Products to Treat COVID-19, Cancer
On April 19, 2023, the FDA issued a warning letter to Medolife Rx D/B/A MedoLife Corp., AELIA Inc. and Queanta Inc.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
News Release
April 14, 2020
ConsumerLab Tests Reveal Best Probiotic Supplements and Those With Quality Issues
White Plains, New York, April 14, 2020 — Probiotic supplements may be helpful for conditions including antibiotic-associated diarrhea, irritable bowel syndrome, and respiratory infections.
Recalls & Warnings
November 11, 2024
FTC Sends Refunds to Customers Who Purchased Sobrenix
On November 7, 2024, the FTC announced it is mailing 56,686 checks totaling more than $536,000 to customers who bought Sobrenix, a supplement sold and marketed by Rejuvica to reduce and eliminate alcohol cravings and consumption.
Clinical Update
Recalls & Warnings
January 28, 2021
FDA Issues Import Alert on Potentially-Dangerous Hand Sanitizers from Mexico
On January 26, 2021, the FDA announced that all alcohol-based hand sanitizers from Mexico will be placed on a countrywide import alert to limit the flow of potentially dangerous products into the US.
Recalls & Warnings
April 24, 2021
Durisan Recalls Hand Sanitizer Due to Microbial Contamination
On April 10, 2021, Durisan announced a recall expansion of Durisan Antimicrobial Hand Sanitizer, NonAlcohol to include non-expired products because they may be contaminated with the bacterium Burkholderia contaminans.
Recalls & Warnings
April 13, 2023
Bountiful to Pay $600,000 to Settle Charges of Amazon “Review Hijacking”
On April 10, 2023, the Federal Trade Commission (FTC) announced it has approved a final consent order against The Bountiful Company (formerly NBTY), whose brands include Nature’s Bounty and Sundown, after the agency charged Bountiful with using deceptive practices to sell its products on ...
Recalls & Warnings
July 20, 2023
FTC Files Complaint Against Company Promoting “Sobrenix” Supplements for Alcohol Cravings
On July 19, 2023, the FTC filed a complaint against Rejuvica and the company owners for promoting its Sobrenix supplements, which contain ingredients such as vitamin B6, thiamin, milk thistle, Chanca Piedra, and dandelion root, to reduce and eliminate alcohol cravings and consumption.
Recalls & Warnings
February 24, 2023
Bountiful Charged with “Review Hijacking,” False Advertising of Nature’s Bounty Supplements on Amazon
On February 16, 2023, the Federal Trade Commission (FTC) charged The Bountiful Company (formerly NBTY), whose brands include Nature’s Bounty and Sundown, with deceptively using Amazon reviews and badges (such as “#1 Best Seller” and “Amazon’s Choice”) from its ...
Recalls & Warnings
January 03, 2025
Essential Oil Sold on Amazon Recalled
On January 2, 2025, Euqee Wintergreen Essential Oils, sold on Amazon were recalled because the packaging is not child-resistant and may pose a risk of poisoning for young children.
Recalls & Warnings
November 23, 2022
CBD Not Permitted in Gummies, Candies, Cookies, Candy, or Pet Treats, Says FDA
On November 16, 2022, the FDA issued warning letters to five companies for selling products such as gummies, tea, cookies, lollipops, fruit snacks, hard candies, and pet treats containing cannabidiol (CBD) and/or delta-8 tetrahydrocannabinol (delta-8 THC) as conventional food products.
Recalls & Warnings
June 27, 2024
“Authentic” Rose Oil Often Is Not
Damask rose essential oil (Rosa × damascene) and products claiming to contain this oil, or “authentic rose oil” may often be adulterated or contain less expensive oils instead, according to a recent bulletin from the American Botanical Association (ABC).
News Release
March 25, 2020
Trouble Sleeping? ConsumerLab Tests Reveal the Best Melatonin Supplements
White Plains, New York, March 25, 2020 — Melatonin supplements can help you fall asleep faster, and some may help you stay asleep.
Recalls & Warnings
April 24, 2023
FTC Warns Almost 700 Companies: Unsupported Health Claims May Result in Substantial Civil Penalties
On April 13, 2023, the Federal Trade Commission (FTC) notified approximately 670 companies that market over-the-counter (OTC) drugs, homeopathic products, dietary supplements, or functional foods that they could incur significant civil penalties if they make unsubstantiated claims about the ...
Recalls & Warnings
October 09, 2023
Organifi Settles Charges of Unsupported Claims
Organifi, LLC has agreed to pay $150,000 in civil penalties and $50,000 in investigative costs as restitution to settle charges that the company made unsupported claims that its Gold, Pure, Green Juice, and Red Juice products could balance hormone and cortisol levels, regulate the ...
Recalls & Warnings
September 01, 2022
FDA Warns Elderberry Fair & Co for Promoting Elderberry Supplements and Apple Cider to Treat Colds & Flu
On August 15, 2022, the FDA issued a warning letter to The Elderberry Fairy & Co., LLC after review of the company’s website found statements about the company’s Elderberry Syrup with Honey, Elderberry Syrup with Agave, and Organic Fire Cider to be drug claims.
Recalls & Warnings
November 30, 2023
Discover Health, LLC Warned for Promoting CBD Products for Cancer, Epilepsy, & More
On November 16, 2023, the FDA issued a Warning Letter to Discover Health, LLC d/b/a Discover CBD and Strain Snobs following a review of the company’s websites that found statements about the company’s Active CBD Oil – Full Spectrum Distillate Cartridge, Active CBD Oil – ...
News Release
March 12, 2020
ConsumerLab Tests Reveal Big Differences Among Elderberry Supplements
White Plains, New York, March 12, 2020 — Elderberry extracts have long been promoted for fighting colds and flu, and, more recently, as a natural remedy for coronavirus (COVID-19).
Recalls & Warnings
April 27, 2023
FTC Returns over $1.1 Million to Consumers for RevMountain Teeth Whitening Products
On April 25, 2023, the FTC announced it will be returning over $1.
Recalls & Warnings
March 23, 2023
FDA Warns Herbal Vitality for Promoting Supplements to Treat Cold & Flu, Kidney Stones & More
On March 7, 2023, the FDA issued a warning letter to Herbal Vitality, Inc.
Recalls & Warnings
June 04, 2021
COVID Antibody and Antigen Tests That May Give False Results Recalled
On May 28, 2021, the FDA warned consumers not to use the Lepu Medical Technology SARS-CoV-2 Antigen Rapid Test Kit and the Leccurate SARS-CoV-2 Antibody Rapid Test Kit (Colloidal Gold Immunochromatography) due to a "high risk of false results when using these tests."
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
Recalls & Warnings
March 02, 2022
Seller of Holistic Tea Warned by FDA
On February 17, 2022, the FDA issued a warning letter to Vital Health & Wellness following an inspection of the company’s website, which found statements made about the company’s product, Dr. Miller’s Holistic Premium Holy Tea, to be drug claims.
Recalls & Warnings
May 05, 2022
Seller of CBD Tinctures, Creams & Pet Products Promoted for Pain, Cancer & More Warned by FDA
On February 11, 2022, the FDA issued a warning letter to Rena’s Organic following a review of the company’s social media and website, which found statements about the company’s 300 mg CBD Full Spectrum Oil Cannabinoid Tincture, 600 mg CBD Full Spectrum Oil Cannabinoid ...
Recalls & Warnings
December 13, 2021
Seller of Vitamin B12 Injections Without Prescription Pleads Guilty
On December 8, 2021, 46-year-old Des Moines resident Bradley Tomlinson plead guilty to a felony charge related to the sale of misbranded vitamin B12 injectable drugs. Tomlinson began selling these injectable drugs in May 2015 and marketed them as weight loss drugs.
Recalls & Warnings
December 01, 2021
FDA Warns Seller of Saffron Supplements
On November 18th, 2021, the FDA issued a warning letter to Saffron Health Sciences following a review of the company's website, social media, and Amazon product listings, which found statements made about the company's products Crocin Rich, Crocin Rich II, and Crocin Rich ...
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
Recalls & Warnings
May 09, 2022
FDA Warns Five Companies for Selling CBD Supplements, Gummies and Creams With Delta-8 THC
On May 4th, 2022, the FDA issued warning letters to five companies for selling CBD and other products labeled as containing delta-8 tetrahydrocannabinol (delta-8 THC).
Recalls & Warnings
May 14, 2021
Maker of Care by Design CBD Settles Charges of Unsupported Claims
On May 5, 2021, Cannacraft, Inc.
Recalls & Warnings
March 04, 2021
Federal Court Bars Confidence USA from Selling Supplements
On March 4, 2021, the FDA announced a permanent injunction has been entered, barring dietary supplement manufacturer Confidence USA Inc. and two of its executives from producing or selling products until they become compliant with current good manufacturing practices (cGMPs).
Recalls & Warnings
February 22, 2021
FDA Warns Seller of Melatonin & Other Sleep Supplements
On February 18, 2021, the FDA issued a warning letter to SANA Group LLC following a review of the company's website, which found statements made about the company's products Sleep Sana Sleep Drops and Sleep Shots to be drug claims.
Recalls & Warnings
February 22, 2021
FDA Warns Sellers of St. John's Wort
On February 18, 2021, the FDA issued warning letters to two companies following reviews of the companies' websites, which found statements made about the companies' St. John's Wort products to be drug claims. These products include St.
Recalls & Warnings
February 02, 2021
FDA Warns Seller of Ginger, Echinacea, and More
On December 10, 2020, the FDA issued a warning letter to Prairie Dawn Herbs following a review of the company's website, which found statements made about the company's products Chest Tea, Ginger Oil, Dandelion root Tincture, Echinacea purpurea Tincture, Antispasmodic Tincture, ...
Recalls & Warnings
July 26, 2023
Five Leaf Pet Botanicals Warned by FDA for Drug Claims
On June 22, 2023, the FDA issued a warning letter to Five Leaf Pet Botanicals, Inc.
Recalls & Warnings
April 25, 2020
Injectable Curcumin, CBD Are Not Safe, Warns FDA
On April 9, 2020, the FDA issued a warning letter to BIOTA Biosciences LLC following a review of the company's website, which found statements made about some of the company's, products, including Canabidiol (CBD) Complex, Cannabidiol+Curcumin, and Curcumin Complex to be ...
Recalls & Warnings
May 23, 2020
BIOTA Biosciences Recalls Injectable CBD and Curcumin Products
On May 20, 2020, BIOTA Biosciences LLC issued a recall of five lots of three injectable products because they were marketed without FDA approval.
Recalls & Warnings
April 28, 2020
Natures CBD Oil Distribution Warned for Joint Pain Claims
On April 20, 2020, the FDA issued a warning letter to Homero Corp DBA Natures CBD Oil Distribution, following an inspection of the company's website which found the company's products, including Natures Pure CBD Oil, CBD Froggies 25mg, CBD Froggies 50mg, CBD Edibles 200mg Frogs, CBD ...
Recalls & Warnings
December 19, 2020
FDA Finds Unapproved Drugs in Many Weight Loss and Sexual Enhancement Products Sold Online
On December 18, 2020, the FDA warned consumers that certain products promoted for weight loss, body building, sexual enhancement, pain relief, sleep, and other uses because may contain harmful ingredients.
Recalls & Warnings
November 24, 2021
FDA Warns Sanitizer Corporation For Manufacturing and Misbranding Violations
On September 24, 2021, the FDA issued a warning letter to Chameleon Beverage Co. Inc.
Recalls & Warnings
April 27, 2022
FDA Tests Find Imported Hand Sanitizer Contains Less Active Ingredient Than Claimed
On April 19th, 2022, the FDA issued a warning letter to Guangzhou Zhongkebaishi Health Industry Co., Ltd.
Recalls & Warnings
July 28, 2022
FDA Warns Three Manufacturers of Sanitizer for Manufacturing and Misbranding Violations
Between July 8 and July 21, the FDA issued warning letters to three companies selling hand sanitizer products that violate Current Good Manufacturing Practices (cGMP), are adulterated and misbranded, and for refusal to allow the agency to access and copy company records.
Recalls & Warnings
May 16, 2020
CBD Oil Recalled, Risk of High Lead Exposure
On May 15, 2020, Summitt Labs issued a recall of one batch of KORE ORGANIC Watermelon CBD Oil Tincture due to contamination with lead. A random sample of this product tested by the Florida Department of Agriculture and Consumer Services found it to contain lead levels at 4.7 ppm.
Recalls & Warnings
April 06, 2022
Mandalorian and Mickey Mouse Hand Sanitizers Recalled Due to Benzene, Methanol
On April 1, 2022, Best Brands Consumer Products, Inc.
Recalls & Warnings
September 08, 2020
Holistic Healthy Pet Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet following a review of the company's website, which found statements made about some of the company's products, including Doctor's Best for Your Pets, Savvy Holistic Health Pet Essences, ...
Recalls & Warnings
March 18, 2021
Hand Sanitizer Recalled Because Packaging Resembles Water Bottle
On March 17, 2021, PNHC, LLC d/b/a Heal the World recalled all lots of Heal the World hand sanitizer because the product is packaged in containers resembling water bottles.
Recalls & Warnings
July 06, 2021
Limar Hand Sanitizer Recalled
On July 1, 2021, Ardil Comercial recalled one lot of Limar Hand Sanitizer packaged because its packaging resembles water bottles which could pose a risk of ingestion.
Recalls & Warnings
June 22, 2021
Prairie Wolf Hand Sanitizer Recalled
On June 21, 2021, Prairie Wolf Spirits, Inc. recalled all lots of Prairie Wolf Distillery hand sanitizer packaged in 16.9 fluid ounce and 20 fluid ounce containers that resemble water bottles because the products pose a risk of ingestion.
Recalls & Warnings
September 08, 2020
FDA Warns Seller of Multivitamin for Manufacturing Violations
On August 14, 2020, the FDA issued a warning letter to Revival Products, Inc.
Recalls & Warnings
August 10, 2020
Gelbac T Antibacterial Handgel Recalled
On August 6, 2020, Incredible Products Sa De Cv recalled Gelbac T Antibacterial Handgel because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
May 12, 2020
FTC Halts Deceptive Supplements & Cosmetics "Free Trial" Scheme
On May 8, 2020, the owners of AH Media Group, LLC agreed to halt their allegedly deceptive practice of luring consumers with supposed "free trial" offers for cosmetics and dietary supplements, then enrolling them in subscriptions and billing them without their consent.
Recalls & Warnings
July 29, 2020
Herbacil Antiseptic Hand Sanitizer Recalled
On July 27, 2020, Broncolin S.A. de C.V. recalled Herbacil Antiseptic Hand Sanitizer 70% Alcohol because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
July 28, 2020
FDA Warns Eskbiochem for Hand Sanitizers That May Contain Toxic Ingredient
On July 23, 2020, the FDA issued a warning letter to Eskbiochem SA de CV which found the company's products, including ClearCare Nogerm ADVANCED HAND SANITIZER and LAVAR GEL HAND SANITIZER, to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
July 17, 2020
Two More Hand Sanitizers That May Contain Toxic Ingredient Recalled
Update: (8/10/20) Soluciones Cosméticas has recalled additional products that may contain methanol, as listed in red below.
Recalls & Warnings
August 04, 2020
Two More Companies Recall Potentially Toxic Hand Sanitizers
Between July 30 and 31, 2020, two more companies recalled hand sanitizers because they may contain methanol, which is toxic when absorbed through the skin or ingested:





