Reviews and Information for Cholestene
Search term may appear only in full report available to members. Join now for full access.
Product Review
Red Yeast Rice Supplements Review
50% of Red Yeast Rice Supplements "NOT APPROVED" in CL Tests

Recalls & Warnings
March 13, 2020
FDA Finds Problems at 52% of Supplement Manufacturing Sites in U.S. and 42% Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2019 (October 1, 2018 - September 30, 2019) of 598 dietary supplement manufacturing facilities in the U.S.
Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Product Review
Nutrition Bars & Cookies Review (For Energy, Fiber, Protein, Meal Replacement, and Whole Foods)
Find the Best Nutrition Bar or Cookie. ConsumerLab Tests Reveals Not All Nutrition Bars and Cookies Contain What They Claim.

Product Review
CBD Oils, Softgels, Gummies, Creams & Salves Review
See How Much CBD and THC We Found in Products.

Clinical Update
7/25/2021
Cholestene Update
The FDA recently advised consumers not to use the popular red yeast rice product, Cholestene, based on its testing. We asked the FDA what it actually found in Cholestene. Find out what we learned in the Update to our Red Yeast Rice Review.
CL Answer
My doctor warned me that red yeast rice can cause liver damage - is that true?
Can red yeast rice cause liver damage? More information on its effects, including possible danger to the liver and kidneys.

Product Review
Collagen Supplements Review
See our Top Picks for Wrinkles and Joints

Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

Product Review
Magnesium Supplements Review (Including Calcium, Vitamins D & K, and Boron)
Find Out What Magnesium Does, Who Needs It, and Our Top Picks Among Supplements
Product Review
Probiotic Supplements Review (Including Pet Probiotics)
Probiotics: See What They Really Contain and Our Top Picks

Product Review
NAD Booster Supplements Review (NAD+/NADH, Nicotinamide Riboside, and NMN)
How Important Is Boosting NAD+ Levels? Find Out and Learn How Booster Supplements Compare.
Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

Product Review
Aloe Juices, Gels, and Supplements Review
How Much Aloe is Really in Aloe Products? Find Out and See Our Top Picks.

Product Review
Plant-Based Milks Review (Almond, Cashew, Coconut, Flax, Hemp, Macadamia, Oat, Pea, and Soy)
Find the Best Non-Dairy Milk Alternative. ConsumerLab Tests Reveal What's Really In Plant-Based Milks.

Clinical Update
10/17/2024
HPF Cholestene Discontinued?
A CL member noted that the red yeast rice supplement HPF Cholestene is no longer sold on Amazon and asked us if it is discontinued. We explain what’s going on in our Red Yeast Rice Supplements Review, which includes our Top Pick for red yeast rice.
Product Review
Iron Supplements Review (Iron Pills, Liquids and Chews)
See Which Iron Supplements Are CL's Top Picks for Different Needs

Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Recalls & Warnings
March 12, 2016
FDA Finds Problems at 58% of Supplement Manufacturing Sites in U.S. and Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2015 (ending September 30) of 483 dietary supplement manufacturing facilities, showing that most -- 58.2% -- received letters indicating noncompliance with current Good Manufacturing Practices (cGMPs).
Product Review
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
Product Review
Cranberry Juices and Supplements Review
CL's Tests Show Which Cranberry Juices and Supplements Are Best and Cost the Least

Product Review
Lactose Intolerance Products Review (Lactase Enzyme Supplements and Lactose-Free Milks)
Choose the Best Lactase Enzyme Supplement and Lactose-Free Milk. Find a CL Approved Lactose Intolerance Product.

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Vitamin C Supplements Review
Find the Best Vitamin C Supplements

Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

Product Review
Green Tea Review: Tea Bags, Loose Leaf Tea, Matcha Powders, and Supplements
Some green teas provide barely any green tea polyphenols, while some others are high strength. See the Test Results and Our Top Picks for Green Tea.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
Black Seed Oil Review
Find Out Which Black Seed Oil Supplements Passed or Failed Our Tests. See Our Top Picks.

Product Review
Sunflower Seeds and Butters Review
High Levels of Toxin Found in Most Sunflower Seeds and Butters

Product Review
Resveratrol Supplements Review (From Red Wine, Knotweed, and Other Sources)
See Which Resveratrol Supplements Were Best In Our Tests and Comparisons. Learn What Resveratrol Can and Can't Do.

Product Review
Coconut Oil and Medium Chain Triglycerides (MCT) Oil Review — Semi-Solid and Liquid Oils & Supplements
Find the Best Coconut Oil and MCT Oil. See How These Oils Compare on Medium Chain Triglycerides (MCTs), Quality, and Value.

Product Review
Reishi Mushroom Supplements Review
Find the Best Reishi Mushroom Supplement. See How Reishi Supplements Differ.

Product Review
Menopause Supplements Review (Soy and Red Clover Isoflavones, Black Cohosh) and Progesterone Creams
Choose the Best Menopause Supplement. Find Out Now Which Soy Isoflavone, Red Clover, Black Cohosh, and Progesterone Products Have the Active Compounds You Want!

Product Review
Lion's Mane and Chaga Supplements Review
Read Labels Carefully -- Many Can Mislead

CL Answer
Where to Safely Buy Real Vitamins and Supplements Online, Not Fakes or Counterfeits
ConsumerLab explains how to avoid counterfeit vitamins and supplements when shopping online on Amazon, Walmart.com, and other sites. Learn to identify authorized sites and sellers and avoid fake supplements. Use the brand-by-brand guide to protect yourself from risk.

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

Product Review
Saffron Supplements Review
See How Saffron Supplements Compare and What They Do

Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

Product Review
Avocado Oil Review
Find the Best Avocado Oil for Purity, Freshness and Taste. Some Others May Include Rotten Avocado or Other Oils.

Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

Product Review
Low-Dose Lithium Supplements Review
Choose the Best Low-Dose Lithium Supplement. CL Tests Reveal Which Low-Dose Lithium Supplements Offer the Best Quality and Value.

Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

Product Review
Elderberry Supplements Review
Find the Best Elderberry Supplement. Tests and Reviews of Popular Elderberry Supplements & CL's Top Picks.

Product Review
Garlic Supplements Review
Find the Best Garlic Supplements. CL Tests Reveal Big Differences in Garlic Strength -- Some Have Little to No Garlic!.

Product Review
Rhodiola Rosea Supplements Review
Do Rhodiola Supplements Help With Depression and Anxiety? Find Out and See Which Rhodiola Supplements Provide the Best Quality & Value.

Product Review
St. John's Wort Supplements Review
Find the Best St. John's Wort Supplement. Only 40% of St. John's Wort Supplements Pass Tests & Strength Varies Widely.

Product Review
SAMe (S-adenosyl-methionine) Supplements Review
Choose the Best SAMe Supplement and Save Money
Product Review
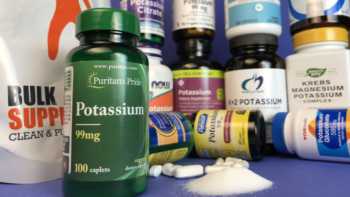
Potassium Supplements Review
Be Careful with Potassium Supplements! Problems Found. Tests and Reviews of Potassium Supplements & CL's Top Picks.
Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

Product Review
Berberine and Goldenseal Supplements Review
Tests Reveal the Best and Worst Berberine and Goldenseal Products

Product Review
Alpha-Lipoic Acid Supplements Review
Choose the Best Alpha-Lipoic Acid Supplement — See the Amounts of Active "R-Isomer" We Found

Product Review
Shelled Walnuts (Halves & Pieces)
See Our Top Picks for Walnuts

Recalls & Warnings
August 16, 2023
FDA Warns Sellers of Homeopathic Products for Infants and Children
On August 9, 2023, the FDA issued a warning letter to ALVA-AMCO Pharmacal Companies, LLC and CalmCo LLC, previously named Ketomi LLC, following a review of the company websites, which found statements about company homeopathic products to be drug claims because they are intended to diagnose, cure, ...
Product Review
Vitamin D Supplements Review (Including Calcium, Magnesium, Vitamin K, and Boron)
Find the Best Vitamin D Supplement and Avoid Problems

Product Review
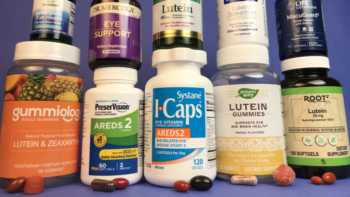
Vision Supplements Review (with Lutein, Zeaxanthin & AREDS2 Formulas)
Find the Best Vision Supplement Based Our Tests
Product Review
Choline and Lecithin Supplements Review (Including Phosphatidylcholine, CDP-Choline, and Alpha-GPC)
Choose the Best Choline Supplement. Find Out How Much Choline Popular Supplements Really Provide.

Product Review
CLA (Conjugated Linoleic Acid) Supplements Review (for Slimming)
Choose the Best CLA Supplement. Not All CLA Supplements Contain What You Expect.

Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

CL Answer
Do "Mr. Happy Stack" supplements improve memory, cognition and mood, and are they safe?
Find out if Happy Stack supplements really work to enhance memory and cognition, plus safety and side effects. ConsumerLab.com's answer explains.
Recalls & Warnings
August 20, 2024
Two Sexual Enhancement Supplements Sold on Amazon Recalled
On August 20, 2024, Veata LLC Endurance Pro Energy Boost capsules and Boulla LLC Boom Max capsules were recalled because they contain sildenafil, a prescription medication which is not permitted in dietary supplements.
Product Review
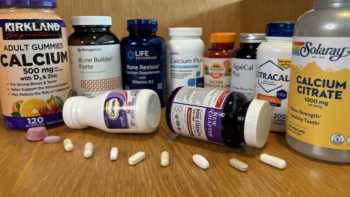
Calcium and Bone Health Supplements Review (Including Vitamins D & K, Magnesium and Boron)
See Which Bone Health Supplements Are Top Picks and Which Fail
Product Review
Green Coffee Bean Extract Supplements Review (for Weight Loss)
Choose the Best Green Coffee Bean Extract. 50% of Green Coffee Bean Extract Supplements Don't Deliver Expected Ingredients.

Product Review
Bilberry Supplements Review
Choose the Best Bilberry Supplement. Some Bilberry Is Not Authentic!

Product Review
DHEA Supplements Review
Choose the Best DHEA Supplement. Beware of Big Differences in Dose and Price.

CL Answer
Does Restore (Biomic Sciences LLC) really improve gut health? What is in Restore?
Learn more about Restore, including clinical studies on gut health, dosage, cost, and safety.

Recalls & Warnings
September 10, 2021
FDA Warns Ten Sellers of "Diabetes" Supplements
On September 7, 2021, the FDA issued warning letters to 10 supplement companies that made drug claims by promoting products to treat diabetes and/or lower blood sugar. Five of the products were sold on Amazon as well as on company websites. The products were promoted with statements such as
Product Review
Maca Supplements Review
Choose the Best Maca Supplement. Make Sure Your Maca Supplement Isn't Contaminated With Lead.

Product Review
Acai Berry Supplements and Beverages Review
Find the Best Acai Berry Supplements and Beverages. See Which Acai Berry Supplements and Beverages Passed Our Tests of Quality.
Product Review
Valerian Supplements Review
Choose the Best Valerian Supplement. Strength and Contamination Vary Widely Among Popular Valerian Brands.

Product Review
L-Tryptophan and 5-Hydroxytryptophan (5-HTP) Supplements Review
Choose the Best L-Tryptophan and 5-HTP Supplements. CL Tests Identify High Quality L-Tryptophan and 5-HTP (5-Hydroxy-L-Tryptophan) at the Best Value.

Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
Product Review
Ashwagandha Supplements Review
Find the Best Ashwagandha Supplement. Only 38% of Ashwagandha Products Pass Tests.

Product Review
Ginkgo (Ginkgo Biloba) Supplements Review
Choose the Best Ginkgo Biloba Supplement. Finding Real Ginkgo Isn't Easy — 60% Fail ConsumerLab's Tests of Quality.

Product Review
Bone Broth Review
Find the Best Bone Broth. Find Out How Much Collagen and Sodium Is Really In Popular Bone Broths.

Recalls & Warnings
November 26, 2019
FDA Warns Companies Selling CBD Products as Dietary Supplements
On November 25, 2019, the FDA issued warning letters to 15 companies for selling products containing CBD (cannabidiol) labeled and marketed as dietary supplements, and/or for making drug claims about these products.
News Release
February 26, 2025
ConsumerLab Survey Shows 21 Most Popular Vitamins and Supplements
White Plains, New York, February 26, 2025 — A recent survey of more than 8,400 people who regularly use dietary supplements shows that among the 212 most popular supplements, those that experienced the greatest absolute growth in popularity during 2024 were biotin (+9.
News Release
February 25, 2025
Top-rated Vitamin and Supplement Brands and Merchants for 2025 Based on Consumer Satisfaction
White Plains, New York, February 25, 2025 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 8,400 responses collected in November/December 2024.
Recalls & Warnings
November 07, 2024
Sexual Enhancement Supplements Sold on Amazon Recalled
On November 4, 2024, four sexual enhancement supplements sold on Amazon and other websites, ZoomMax and ZapMax (Boulla LLC) and VitalityXtra and PeakMax (VitalityVita LLC) were recalled because they were found to contain undeclared prescription drugs, ...
Recalls & Warnings
July 21, 2020
51 CBD Products Recalled Due to Lead Contamination
On June 23, 2020, InHe Manufacturing, LLC and MHR Brands issued a recall of fifty-one CBD products due to contamination and/or potential with lead. Thirty-six of the products are marketed for people and fifteen of the products are marketed for pets.
Recalls & Warnings
April 25, 2022
Some OTC Skin Lighteners Contain Potentially Harmful Ingredient, Warns FDA
On April 19th, 2022, the FDA issued warnings to 12 companies for selling over-the-counter (OTC) skin-lightening products containing hydroquinone.
Recalls & Warnings
June 24, 2024
Canned Coffee Recalled Due to Botulism Risk
On June 17, 2024, Snapchill LLC recalled its canned coffee products because they have the potential to grow the toxic bacterium Clostridium botulinum.
Product Review
Huperzine A Supplements Review
Choose the Best Huperzine A Supplement. CL Tests Reveal the Best Huperzine A Supplements for Memory.

Product Review
Potassium Iodide (KI) and Iodate (KIO3) Radioprotective Pills Review
Learn How to Choose the Best Radioprotective Iodine Pill and Use It Properly
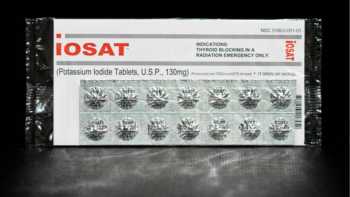
CL Answer
Should you take olive oil as a supplement?
Although extra virgin olive oil has many possible health benefits, such as reduced risk of heart disease and improved blood sugar control, these and other benefits have been demonstrated when olive oil replaces for saturated fats in the diet, not when taken as a supplement, as we explain.

Recalls & Warnings
April 23, 2019
"Brain Boosting" Supplements Were Promoted With Non-Existent Clinical Studies
On April 10th, 2019, the FTC (Federal Trade Commission) announced the marketers of cognitive enhancement supplements Geniux, Xcel, EVO, and Ion-Z have agreed to settle charges that they made false claims about the product, including fake research references and celebrity ...
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Recalls & Warnings
July 14, 2022
FDA Warns Company Selling Supplements with Dangerous Steroid-like Substances
On July 6, 2022, the FDA issued a warning letter to Elite Supplement Center LLC and Elite Supplement Training Facility LLC for selling the following products labeled as containing steroid-like substances known as selective androgen receptor modulators (SARMs), which are not permitted in dietary ...
CL Answer
What are the side effects of red yeast rice?
Find out which side effects can be caused by red yeast rice supplements that are taken to lower cholesterol levels. ConsumerLab.com's answer explains.

Recalls & Warnings
May 12, 2020
FTC Halts Deceptive Supplements & Cosmetics "Free Trial" Scheme
On May 8, 2020, the owners of AH Media Group, LLC agreed to halt their allegedly deceptive practice of luring consumers with supposed "free trial" offers for cosmetics and dietary supplements, then enrolling them in subscriptions and billing them without their consent.
Recalls & Warnings
April 28, 2020
Ten Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On April 24, 2020, the FTC announced that it sent warning letters to ten multi-level marketing companies for selling products such as essential oils and immune system boosters with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrespresenting potential earnings people ...
Recalls & Warnings
July 31, 2020
FDA Warns Seven Sellers of "Hangover Cures"
The FDA recently issued warning letters to seven companies for promoting hangover relief products with drug claims (use the links below to read the full warning letter):
Recalls & Warnings
November 18, 2024
Manufacturer of Grandma’s Herbs Kidney Warned by FDA
On October 20, 2023, the FDA issued a warning letter to Top Health Manufacturing, LLC, manufacturer of Grandma’s Herbs Kidney supplement, following an inspection of the company’s facility that found its products to be adulterated because they were, packed, labeled or held under ...
Recalls & Warnings
September 24, 2024
Manufacturer of Mushroom Gummies Warned by FDA for Violations
On August 13, 2024, the FDA issued a Warning Letter to Restorative Botanicals, LLC, following an inspection of the company’s facility found the company’s products, including its My GUT Shrooms Gummies and My IMMUNE Shrooms Capsules, to be adulterated because they were prepared, ...
Recalls & Warnings
February 03, 2025
Four Male Enhancement and Energy Supplements Found to Contain Drugs
On January 16, 2025, the FDA issued a Warning Letter to Mihon Corp. d/b/a VitalityVita and Boulla, LLC after FDA analysis found four of the company’s supplements, VitalityXtra, PeakMax, ZapMax, and ZoomMax, to contain undeclared sildenafil.
News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
News Release
February 25, 2023
Top-rated Vitamin and Supplement Brands and Merchants for 2023 Based on Consumer Satisfaction
White Plains, New York, February 25, 2023 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,600 responses collected in November/December 2022.
News Release
February 24, 2023
Probiotics Rise in Popularity as Vitamin C, Melatonin, and Others Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 25, 2023 —A recent survey of 8,600 people who regularly use dietary supplements shows that probiotics (+3.04 percentage points), quercetin (+2.3 pts), and vitamin K (+1.
News Release
May 26, 2022
50% of Red Yeast Rice Supplements Fail ConsumerLab Tests
White Plains, New York, May 26, 2022 — Red yeast rice naturally contains cholesterol-lowering lovastatin compounds. However, recent ConsumerLab tests of popular red yeast rice products on the market revealed many did not contain any detectable lovastatin.
News Release
February 25, 2022
Top-rated Vitamin and Supplement Brands and Merchants for 2022 Based on Consumer Satisfaction
White Plains, New York, February 25, 2022 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,049 responses collected in November/December 2021.
CL Answer
What are the health benefits of tart cherry juice?
See the evidence for tart cherry juice health benefits from clinical studies. Find out if tart cherry has anti-inflammatory effects, if it can improve sleep, lower high blood pressure, or help for muscle pain and osteoarthritis.

Recalls & Warnings
December 26, 2023
Total Body Nutrition, TBN Labs, and Loud Muscle Science Banned from Selling Adulterated and Misbranded Dietary Supplements
On December 11, 2023, Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science LLC, as well as the companies’ owner, Mohammed Islam, were prohibited by federal court from manufacturing and distributing adulterated and misbranded dietary supplements.
CL Answer
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
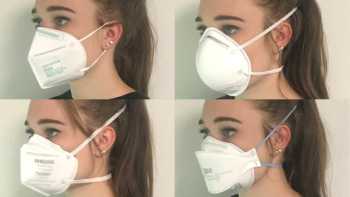
Recalls & Warnings
August 16, 2023
FDA Warns Hekma Center, LLC for Promoting Products to Treat Anemia, Diabetes, Depression, & More
On June 2, 2023, the FDA issued a warning letter to Hekma Center, LLC following review of the company’s website and social media, which found statements about the company’s Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa ...
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
April 29, 2015
FDA Targets Weight Loss and Workout Supplements Listing Synthetic Stimulant DMBA
On April 24, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called 1,3-dimethylbutylamine (DMBA) on product labels.
Recalls & Warnings
April 02, 2021
Three Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 24 and 26, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and tadalafil.
Recalls & Warnings
April 02, 2021
FDA Warns Sellers of Prostate, Reishi, Immune Products, and More
On March 16, 2021, the FDA issued warning letters to two companies following reviews of their websites which found statements made about the companies' products to be drug claims.
Recalls & Warnings
April 17, 2020
Joint Pain Supplement Isoprex Settles Charges of Making False Claims
On April 16, 2020, Renaissance Health Publishing, LLC, agreed to halt their allegedly deceptive advertising claims about their Isoprex supplement that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
April 14, 2020
FTC Warns Companies Selling Immune "Boosters," Vitamin C and More for Coronavirus Claims
On April 14, 2020, the FTC announced that it has sent warning letters to ten companies for selling products such as immune boosters, silicone facial brushes, air purifiers, and intravenous vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
January 11, 2023
Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 9, 2022, the FDA issued a warning letter to Distributor RFR, LLC after laboratory analysis of the company’s SANGTER Natural Male Energy Supplement found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
January 19, 2023
Male Sexual Enhancement Supplement Adam’s Secret Found to Contain Prescription Medication
On January 10, 2023, the FDA issued a warning letter to HIS Enterprise Inc dba Adam’s Secret USA, LLC after laboratory analysis found Adam’s Secret Extra Strength 3000 Platinum, Adam’s Secret Extra Strength Blue, Adam’s Secret Extra Strength Purple, Adam's Secret ...
Recalls & Warnings
November 28, 2022
Seller of Elderberry, Tea Warned for Claims of Treating Cold, Flu, Cancer
On October 18, 2022, the FDA issued a warning letter to Rosebud’s Ranch and Garden, LLC after inspection of the company’s website and social media found statements about the company’s Domestic Divas – Colds and Flu (Tea), Domestic Divas - No Pain No Gain Tea, Green ...
Recalls & Warnings
December 12, 2022
FDA Warns Saffron USA for Promoting Teas to Treat Insomnia, Osteoporosis & Cancer
On September 23, 2022, the FDA issued a warning letter to Saffron USA LLC following inspection of the company’s website which found statements about its Allergy Blend, Chamomile Tea Petals, Diabetic Support Blend, Orange Blast Tea, and Saffron Loose Tea products to be drug ...
Recalls & Warnings
September 29, 2022
Sexual Enhancement Supplement Sold on Amazon and Walmart Recalled Due to Undeclared Drugs
On September 27, 2022, Proper Trade LLC/My Stellar Lifestyle recalled two lots of Wonder Pill after Amazon laboratory analysis found the product to contain undeclared tadalafil, a prescription medication.
Recalls & Warnings
August 03, 2022
Sexual Enhancement Supplement Recalled Due to Sildenafil
On August 1, 2022, DISTRIBUTOR RFR, LLC recalled one lot of SANGTER Energy Supplement 3000 mg to the consumer level after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
August 08, 2022
Seller of CBD Warned for COVID-19 Claims
On August 4, 2022, the FDA sent a warning letter to FluxxLab LLC following a review of the company’s website and social media which found statements about the company’s Covid-19 Immune Support Tincture and CBDA+CBD Oil Tincture products to be drug claims because they ...
Recalls & Warnings
September 01, 2022
FDA Warns Elderberry Fair & Co for Promoting Elderberry Supplements and Apple Cider to Treat Colds & Flu
On August 15, 2022, the FDA issued a warning letter to The Elderberry Fairy & Co., LLC after review of the company’s website found statements about the company’s Elderberry Syrup with Honey, Elderberry Syrup with Agave, and Organic Fire Cider to be drug claims.
Recalls & Warnings
July 13, 2022
FDA Warns Sellers of Tainted Honey-Based Sexual Enhancement Products
On July 12, 2022, the FDA issued warning letters to four companies selling honey-based products promoted for sexual enhancement after tests conducted by the FDA found the products to contain the prescription drugs Tadalafil and Sildenafil.
Recalls & Warnings
February 13, 2019
Supplements Promoted for Alzheimer's Disease and Dementia Sell "False Hope," Warns FDA
On February 11, 2019, the FDA warned consumers to beware supplements promoted to prevent or treat Alzheimer's disease or dementia.
Recalls & Warnings
May 11, 2006
Weight-Loss Marketers Pay $3 Million for Deceptive Advertising
On May 11, the Federal Trade Commission (FTC) announced that sellers making questionable weight-loss and fat-loss claims to peddle skin gels and diet supplements will pay $3 million to settle charges that their deceptive claims violated federal law.
Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Recalls & Warnings
December 15, 2020
FDA Warns JC Ayur Life LLC for Drug Claims
On October 29, 2020, the FDA issued a warning letter to JC Ayur Life LLC following a review of the company's website, which found statements made about the company's product Heritage of Ayurveda Dia-Tonic Incudil Herbal Dietary Supplement to be drug claims.
Recalls & Warnings
November 20, 2020
FTC Files Complaint Against Two Supplement Companies for Deceptive Marketing
On November 20, 2020, the FTC approved a Part 3 administrative complaint against Health Research Laboratories, LLC, its owner Kramer Duhon, and Whole Body Supplements, LLC for making unverified claims that their products can prevent or treat diseases.
Recalls & Warnings
September 01, 2020
Custom Nutraceuticals, LLC Warned for Manufacturing Violations
On August 6, 2020, the FDA issued a warning letter to Custom Nutraceuticals, LLC, following a facility inspection which found the company's products, including Thermal Revolution Black, Anabolic Blackout Raspberry Lemonade, Militia Re-COMP, and Rhino Rampage Wildberry to be ...
Recalls & Warnings
November 30, 2023
Discover Health, LLC Warned for Promoting CBD Products for Cancer, Epilepsy, & More
On November 16, 2023, the FDA issued a Warning Letter to Discover Health, LLC d/b/a Discover CBD and Strain Snobs following a review of the company’s websites that found statements about the company’s Active CBD Oil – Full Spectrum Distillate Cartridge, Active CBD Oil – ...
CL Answer
When taking a statin drug like Lipitor or Crestor, are there supplements I should avoid or take?
Learn about the interactions between certain supplements and atorvastatin (Lipitor), rosuvastatin (Crestor), and other cholesterol-lowering statins.
CL Answer
Where to Safely Buy Real Water Filters Online, Not Fakes or Counterfeits
ConsumerLab explains how to avoid counterfeit water filters when shopping online on Amazon, Walmart.com, and other sites. Learn to identify authorized sites and sellers and avoid fake filters. Use the brand-by-brand guide to protect yourself from risk.

Recalls & Warnings
April 03, 2024
FDA Warns Ambaya Gold for Promoting Products for Depression, Cancer, & Arthritis
On December 5, 2023, the FDA issued a Warning Letter to Ambaya Gold Health Products, LLC following review of the company’s website and social media, which found statements about the company’s Brain Balance, Immune System Boost, Dentist In A Bottle, Essensiac, Fulvic Green, Silver ...
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
January 13, 2022
Senna Laxative Recalled Due to Microbial Contamination
On January 11, 2022, Lohxa LLC issued a voluntary recall of one lot of Senna Syrup 8.
Recalls & Warnings
April 16, 2019
DMHA and Phenibut Are Not Permitted in Dietary Supplements, Warns FDA
On April 16, 2019, the FDA announced it has issued 11 warning letters to companies whose dietary supplement products contain the drugs DMHA or phenibut, and therefore are in violation of the law.
Recalls & Warnings
November 23, 2022
CBD Not Permitted in Gummies, Candies, Cookies, Candy, or Pet Treats, Says FDA
On November 16, 2022, the FDA issued warning letters to five companies for selling products such as gummies, tea, cookies, lollipops, fruit snacks, hard candies, and pet treats containing cannabidiol (CBD) and/or delta-8 tetrahydrocannabinol (delta-8 THC) as conventional food products.
Recalls & Warnings
December 05, 2024
Liquid Multivitamin Recalled
On November 6, 2024, Armonia Natural Store LLC issued a recall of 64 bottles of OneMultivitaminic GAF-PLUS Advance 300 ml because they contain undeclared acetaminophen and dexamethasone phosphate (which are not permitted in dietary supplements) and the undeclared sweetener aspartame.
Recalls & Warnings
December 19, 2024
Toxic Herb Found in More Weight Loss Supplements
On December 13, 2024, Motivate Me Ashley, LLC recalled the following VidaSlim brand products: VidaSlim 90-day (Original Root, Root Plus, and Root Capsules), VidaSlim 30-day (Original Root, Root Plus, and Root Capsules), VidaSlim 7-day Sample Size (Original Root, Root Plus, and ...
Recalls & Warnings
September 12, 2024
Root Bioscience Warned for CBD Claims
On August 30, 2024, the FDA issued a warning letter to Root Bioscience Brands, LLC dba Naternal following a review of the company's websites, which found statements made about the company's products, including CBD Oils Move CBD+CBG Oil, Rest CBD+CBN Oil, Nurture Broad Spectrum ...
Recalls & Warnings
September 23, 2024
Lactaid Milk Recalled Due to Allergen
On September 20, 2024, HP Hood LLC voluntarily recalled five SKUs of refrigerated Lactaid Milk due to the product potentially containing trace amounts of almond, which is not declared on the label. No illness has been reported to date.
Recalls & Warnings
September 24, 2024
Supplement for Eczema Recalled
On September 18, 2024, 123Herbals LLC issued a recall for all lots of Vail-Bon Jie Yang Wan capsules, which are promoted to treat eczema and other skin conditions, because they contain undeclared dexamethasone and chlorpheniramine.
Recalls & Warnings
February 27, 2025
Male Enhancement Supplement Sold on Amazon and Walmart Recalled
On February 25, 2025, Natural Dior LLC recalled affected lots of a dietary supplement Vitafer-L Gold Liquid because it contains undeclared tadalafil, which is not permitted in dietary supplements.
Recalls & Warnings
March 10, 2025
Probiotics and Prebiotic Gummies Recalled
On January 17, 2025, Health and Happiness (H&H) LLC.
Recalls & Warnings
March 13, 2025
FTC Sends Refund Checks to Consumers of Pure Green Coffee Supplement
On March 6, 2025, the Federal Trade Commission (FTC) announced it is mailing 39,977 checks totaling more than $905,000 to consumers who purchased Pure Green Coffee, to settle charges the product was promoted with deceptive health claims and marketing practices.
Recalls & Warnings
April 17, 2024
FDA Warns Lipari Foods for Mislabeled Walnuts, Other Violations
On April 9, 2024, the FDA issued a Warning Letter to Lipari Foods Operating Company, LLC following multiple complaints and subsequent product recalls in August and September of 2023, which found the company did not follow the requirements of Current Good Manufacturing Practice (CGMP), Hazard ...
Recalls & Warnings
April 24, 2024
Organic Basil Sold at Trader Joe’s & Other Markets Recalled Due to Salmonella Risk
On April 18, 2024, Infinite Herbs, LLC issued a recall of certain packages of Infinite Herbs and Melissa’s brand fresh organic basil due to the potential presence of Salmonella.
News Release
February 24, 2022
Consumers Returned to Pre-Pandemic Supplement Usage in 2021, ConsumerLab Survey Reveals
White Plains, New York, February 24, 2022 —A survey of 8,049 people who use dietary supplements shows many supplements that declined in use in 2020 began bouncing back in 2021, such as magnesium (+2.4 percentage points), and CoQ10 (+2.7 pts).
Recalls & Warnings
October 12, 2020
NutraClick to Pay $1.04 Million for Illegally Billing Consumers
On September 22, 2020, the Federal Trade Commission (FTC) announced that NutraClick LLC agreed to pay $1.04 million to settle FTC charges that the company was deceptively selling and billing consumers for supplements and beauty products.
Recalls & Warnings
June 09, 2020
Six More Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On June 5, 2020, the FTC announced that it sent warning letters to six multi-level marketing companies for selling products such as immune system boosters and probiotics with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrepresenting potential earnings people who have ...
Recalls & Warnings
November 30, 2023
Original The Rock Capsules Recalled Due to Undeclared Sildenafil
On October 18, 2023, Noah’s Wholesale, LLC issued a nationwide recall of one lot of the company’s Original The Rock capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Recalls & Warnings
January 15, 2024
Suprex Carb & Sugar Block Recalled
On December 7, 2023, Vita 360, LLC issued a recall of one lot of SUPREX Plant Based Nutrition Carb & Sugar Block after FDA analysis found it to contain only 16 mcg of chromium per serving and not 100 mcg of chromium per serving, as listed on the label.
Recalls & Warnings
January 15, 2024
5 Star Nutrition Will Pay $4.5 Million for Selling Misbranded Workout Supplements
On January 12, 2024, Defyned Brands, also known as 5 Star Nutrition LLC, pleaded guilty in federal court to three-counts of distributing misbranded dietary supplements following an investigation by the Food and Drug Administration’s Office of Criminal Investigations (FDA-OCI).
Recalls & Warnings
July 24, 2023
ONO Overnight Oats Recalled Due to Allergen Risk
On July 18, 2023, ONO LLC issued a voluntary recall of the company’s ONO Vegan Blueberry Muffin Protein Overnight Oats due to undeclared milk.
Recalls & Warnings
August 03, 2023
Ozona Organics Liquid Probiotics Recalled Due to Risk of Microbial Contamination
On August 1, 2023, Ozona Organics, LLC recalled certain lots of its probiotic supplement, Ozona Probiotics for Digestive Health, also labeled as GoHealthy Probiotics for Infants, Toddlers, and Kids and GoHealthy Probiotics for Infants, Kids, Men, and Women, due to the ...
Recalls & Warnings
April 03, 2023
Smoked Salmon Recalled Due to Listeria Risk
On March 14, 2023, Seven Seas International USA, LLC issued a voluntary recall of 295 cases of Biltmore Smoked Sockeye Salmon after routine testing by the Florida Department of Agriculture and Consumer Services discovered the presence of Listeria monocytogenes or Salmonella.
Recalls & Warnings
October 09, 2023
Orgain Protein Powder Recalled Due to Allergen Risk
On October 4, 2023, Orgain LLC issued a recall of four lots of the company’s Organic Protein Powder + Superfoods, Creamy Chocolate Fudge flavor protein powder after a co-manufacturer informed the company that the product contained undeclared sesame, which is now one of nine food ...
Recalls & Warnings
December 22, 2021
FDA Warns Seller of Liquid Magnesium, B-12, Berberine & More
On December 9, 2021, the FDA issued a warning letter to Wholly Liquid Nutritional Supplements LLC because it found statements on the company's website and social media about its products, including SpiroLaze, BioLaze, LiquiLaurin, VIT-B12, DeStress, and Omega Plus, to be ...
Recalls & Warnings
December 09, 2021
FDA Warns Seller of Curcumin, Lion's Mane, Quercetin & More
On November 9, 2021, the FDA issued a warning letter to Synaptent, LLC because it found statements on the company's website about its products, including Berberine HCL, Curcumin, Lion's Man, Milk Thistle, Quercetin, Boswellia Serrata Extract, and Garlic Extract to be ...
Recalls & Warnings
May 29, 2021
FDA, FTC Warns Five Sellers of "Infertility" Supplements
The FDA and FTC (Federal Trade Commission) sent warning letters to the following five companies in May for illegally selling dietary supplements promoted with claims to treat infertility and other reproductive health issues:
Recalls & Warnings
April 23, 2021
FDA Warns 5 Sellers of Unapproved COVID-19 Tests
Between March 18 and April 6, 2021, the FDA issued warning letters to five companies for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
Recalls & Warnings
January 04, 2024
Toxic Herb Found in More Tejocote Root Supplements
On January 3, 2024, the FDA warned consumers not to purchase or use certain tejocote root supplements after FDA laboratory analysis confirmed the products contain yellow oleander (Thevetia peruviana), a toxic herb.
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
News Release
February 25, 2021
Top-rated Vitamin and Supplement Brands and Merchants for 2021 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2021 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,647 responses collected in November 2020.
News Release
March 03, 2020
Best and Worst Chia Seed Products Revealed by ConsumerLab
White Plains, New York, March 3, 2020 — Chia seeds are rich in fiber and a good source of healthful oils, particularly the omega-3 fatty acid ALA (alpha-linolenic acid), as well certain vitamins and minerals.
Recalls & Warnings
September 04, 2018
Federal Court Shuts Down Maker of Sexual Enhancement Products
On August 30, 2018, the FDA announced that U.S.
Recalls & Warnings
November 03, 2022
Seller of CBD Warned for COVID-19 Claims
On November 1, 2022, the FDA issued a warning letter to Alternative Health Distribution LLC (d/b/a CannaAid) following a review of the company’s website, which found statements about the company’s cannabidiol (CBD) products to be drug claims.
Recalls & Warnings
November 10, 2022
Adam’s Polishes Hand Sanitizer Recalled Due to Toxic Methanol
On November 5, 2022, Adam’s Polishes, LLC issued a nationwide recall of 20 lots of Adam’s Polishes Hand Sanitizer following FDA testing, which found the presence of methanol in one lot.
Recalls & Warnings
November 21, 2022
6 Supplement Companies Warned by FDA for Making Cholesterol Claims
On May 4, 2022, the FDA issued warning letters to six supplement companies following review that found statements made on company websites and Walmart purchase pages suggesting the products could lower cholesterol to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
July 26, 2022
Sexual Enhancement Supplement Sold on Amazon Recalled
On July 21, 2022, Ultra Supplements LLC issued a recall of one lot of Sustango capsules after Amazon laboratory analysis found the presence of the prescription drug Tadalafil. The company has received no reports of adverse effects related to this recall to date.
Recalls & Warnings
March 22, 2023
Of Concern: The Daily Post and Hiya
White Plains, New York, March 22, 2023 — An "advertorial" for Hiya Kids Daily Multivitamin appearing on the website "The Daily Post" provided misinformation and suggested that ConsumerLab.com recommended this product, which is completely false.
Recalls & Warnings
January 26, 2023
Allergy Bee Nasal Swabs Contaminated With Illness-Causing Bacteria
On January 18, 2023, the FDA issued a warning letter to Buzzagogo, LLC, after the company’s Allergy Bee Gone for Kids nasal swab products were found to be contaminated with bacteria that have the potential to cause life-threatening illness.
Recalls & Warnings
January 31, 2023
Over $973,000 Returned to NutraClick Consumers
On January 25, 2023, the FTC announced it will be returning over $973,000 to over 17,000 consumers who lost money after NutraClick LLC allegedly automatically enrolled them in unwanted membership programs for supplements and beauty products.
Recalls & Warnings
December 19, 2022
High Impact Plant Protein Powder Recalled
On December 15, 2022, THGH Partners LLC issued a recall of one lot of its High Impact Plant Protein due to the presence of undeclared milk.
Recalls & Warnings
April 27, 2022
FDA Warns Manufacturer of Topical Antiseptic Products for COVID Claims
On April 19th, 2022, the FDA issued a warning letter to Kleenhanz, LLC following a review of the company’s website and social media which found statements about the company’s Kleenhanz Towelettes topical antiseptic products to be drug claims.
Recalls & Warnings
July 06, 2022
Two Companies Banned From Selling Supplements to Treat Heart Disease, Neuropathy
On June 30, 2022, the Federal Trade Commission (FTC) finalized an administrative complaint order against two Texas-based companies, Health Research Laboratories, LLC and Whole Body Supplements, LLC, for making unverified claims that their products can prevent or treat disease.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Recalls & Warnings
July 01, 2022
Male Enhancement Supplements Sold on Amazon Recalled
On January 27, 2022, Loud Muscle Science LLC issued a voluntary recall of various lots of Launch Sequence supplements because they were found to contain the prescription drug Tadalfil.
News Release
February 29, 2020
Collagen and Magnesium Rise in Popularity, as Fish Oil and Curcumin Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 29, 2020 — A recent survey of 9,782 people who use dietary supplements shows that collagen (+ 4.1 percentage points), magnesium (+ 2.3 pts) and CBD (+ 2.
News Release
February 25, 2020
Top-rated Vitamin and Supplement Brands and Merchants for 2020 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2020 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,782 responses collected in late November and early December 2019.
Recalls & Warnings
April 21, 2011
FTC Targets Fake News Sites Making Deceptive Acai Claims
On April 19, 2011, the Federal Trade Commission (FTC) requested federal courts to temporarily halt the allegedly deceptive tactics of 10 operations using fake news websites to market acai berry weight-loss products.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
September 08, 2020
FDA Warns Seller of Opioid Withdrawal Supplement
On August 25, 2020, the FDA issued a warning letter to Renewal Supplements LLC following a review of the company's website, which found statements made about the company product Opi-Cure to be drug claims.
Recalls & Warnings
May 09, 2020
FTC Warns 45 More Companies for Coronavirus Claims
On May 7, 2020, the FTC announced that it sent warning letters to 45 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 07, 2023
Belmont Eyecare Warned for Colloidal Silver, MSM, and Castor Oil Eye Drop Claims
On December 1, 2023, the FDA issued a Warning Letter to Belmont Eyecare LLC, following a review of the company’s website, which found statements about the company’s Colloidal Silver Eye Drops, MSM Eye Drops, Organic Daytime Oil Eye Drops, Organic Daytime Oil Eye Drops Small, Organic ...
News Release
August 22, 2019
Best Reishi Mushroom Supplements Identified by ConsumerLab
White Plains, New York, August 22, 2019 — Reishi mushroom supplements are promoted for many uses, from strengthening the immune system and lowering blood sugar, to improving cardiovascular health and reducing cancerous tumors.
News Release
July 31, 2019
ConsumerLab Tests Reveal Best CoQ10 and Ubiquinol Supplements
White Plains, New York, July 31, 2019 — CoQ10 is among the most popular supplements, commonly taken to offset a decline in natural levels of CoQ10 that can occur with the use of statin medications, decrease statin side effects, and increase energy.
News Release
July 10, 2019
Best Selenium Supplements Identified by ConsumerLab
White Plains, New York, July 10, 2019 — Selenium is an essential mineral important for proper immune and thyroid function but taking a selenium supplement is often not necessary and, in some people, may increase the risk of cancer or diabetes.
News Release
June 25, 2019
ConsumerLab Tests Reveal Best B Vitamin Supplements -- 19% of B Vitamin Supplements Fail CL's Tests of Quality
White Plains, New York, June 25, 2019 — B vitamins and complexes are among the most popular supplements sold in the U.S. because B vitamins are essential for a wide range of functions in the body.
News Release
May 15, 2019
ConsumerLab Tests Reveal Big Differences in Digestive Enzyme Supplements
White Plains, New York, May 15, 2019 — Digestive enzyme supplements can help improve digestion and the absorption of nutrients, and may reduce symptoms of indigestion. But to work, they must provide a certain amount of enzyme activity.
News Release
April 25, 2019
Best Coconut Water? ConsumerLab Tests Popular Products, Reveals Top Pick
White Plains, New York, April 25, 2019 — Coconut water is often promoted as a healthy way to stay hydrated and a natural alternative to sports drinks.
News Release
April 01, 2019
Not All Quercetin Supplements Contain What They Claim, ConsumerLab Tests Reveal
White Plains, New York, April 1, 2019 — Quercetin is a flavonoid found in foods such as onions, kale and apples. Sold as a supplement, quercetin is promoted to help with a range of conditions including prostatitis, asthma, and rheumatoid arthritis, as well as blood sugar control.
News Release
March 11, 2019
Best NAC (N-acetyl cysteine) Supplements Identified by ConsumerLab
White Plains, New York, March 11, 2019 — NAC (N-acetyl cysteine) supplements are promoted for many uses, including "liver support," "immune support," and reducing symptoms of the flu and flare-ups of chronic bronchitis.
News Release
March 07, 2019
Best Coconut and MCT Oils Identified by ConsumerLab
White Plains, New York, March 7, 2019 — Coconut oil is often promoted as a "healthy fat" and an alternative source of energy to help with weight loss and in conditions such as Alzheimer's disease because it contains medium chain triglycerides (MCTs).
News Release
February 25, 2019
Top-rated Vitamin and Supplement Brands and Merchants for 2019 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2019 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,931 responses collected in late November and early December 2018.
News Release
August 24, 2018
Few Red Yeast Rice Supplements Provide Enough Cholesterol-Lowering Compounds to Likely Be Effective, ConsumerLab Tests Reveal
White Plains, New York, August 24, 2018 — Research shows that red yeast rice, which contains naturally-occurring lovastatin compounds, can lower "bad" LDL cholesterol.
News Release
February 25, 2018
Top-rated Vitamin and Supplement Brands and Merchants for 2018 Based on Consumer Satisfaction
White Plains, New York, February 25, 2018 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,446 responses collected in late November and early December 2017.
News Release
May 23, 2011
Red yeast rice supplements weaker now than in 2008; Wide variation among brands and contamination discovered by ConsumerLab.com -- Popular cholesterol-lowering supplements tested and compared
WHITE PLAINS, NEW YORK — MAY 23, 2011 — ConsumerLab.com announced today that tests of eleven red yeast rice supplements revealed enormous differences in levels of cholesterol-lowering statin compounds. Statin levels fell dramatically among brands previously tested in 2008.
News Release
July 01, 2008
Tests of ten red yeast rice supplements by ConsumerLab.com reveal significant statin levels, but some pills contaminated -- Popular cholesterol-lowering supplements compared in new report
WHITE PLAINS, NEW YORK — JULY 1, 2008 — ConsumerLab.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
April 10, 2021
Three More Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 30 and April 5, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
July 06, 2021
CBD Products Were Promoted to Treat Cancer and Alzheimer's Without Proof, Says FTC
On July 6, 2021, the Federal Trade Commission (FTC) announced that it has approved a final administrative consent orders against Kushly Industries LLC and the company's owner, Cody Alt, for allegedly making unsupported health claims about its CBD products.
Recalls & Warnings
June 08, 2021
FDA Warns Seller of "CoronaBox" Containing Vitamin D, Probiotics & More for Unsupported Claims
On May 24, 2021, the FDA issued a warning letter to Everything Health LLC following a review of the company's website by the FDA and Federal Trade Commission (FTC) which found the company promoted its CoronaBox (which contains cordyceps, vitamins, and K2, magnesium, ginger, probiotics ...
Recalls & Warnings
December 09, 2021
Florida Man Convicted for Distributing Steroids Labeled as Dietary Supplements
On December 9, 2021, 37-year-old Florida resident James Boccuzzi was convicted of one count of conspiracy to defraud the U.S. Food and Drug Administration (FDA) and one count of conspiracy to distribute controlled substances.
Recalls & Warnings
November 09, 2021
Five Brands of Protein Supplements Recalled Due to Allergen Risk
On November 9, 2021, Nutracap Holdings, LLC issued a recall of certain Boba Origin, Etedream, RAW, Steel, and Vital Force protein supplements because they contain potential allergens, including soy, milk, wheat, and/or coconut, that are not declared on the label.
Recalls & Warnings
September 14, 2021
Seller of Lion's Mane, Other Mushroom Supplements Warned by FDA
On July 20, 2021, the FDA issued a warning letter to Brilliant Enterprises LLC because it found statements made on the company's website and social media about mushroom supplements, including Lion's Mane, BoomChaga, CordaCex, and LVL:MAX to be drug claims.
Recalls & Warnings
August 31, 2004
Two Makers of Weight Loss and Sex Enhancement Supplements Stopped From Making Unsubstantiated Claims
On August 27, 2004, the Federal Trade Commmission (FTC) reported that two Maine-based dietary supplement marketers and their principals have agreed to settle FTC charges that they made deceptive advertising claims about their dietary supplement products, in violation of federal law.
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
Recalls & Warnings
August 08, 2022
Recall of Oral Magnesium Laxatives Expanded
On August 3, 2022, Plastikon Healthcare, LLC issued a recall of multiple oral suspension products due to microbial contamination.
Recalls & Warnings
May 09, 2022
FDA Warns Five Companies for Selling CBD Supplements, Gummies and Creams With Delta-8 THC
On May 4th, 2022, the FDA issued warning letters to five companies for selling CBD and other products labeled as containing delta-8 tetrahydrocannabinol (delta-8 THC).
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
Recalls & Warnings
July 18, 2022
CVS, Target, Walgreens, and Other Oral Magnesium Citrate Laxatives Recalled Due to Bacterial Contamination
On July 14, 2022, Magnesium Citrate Laxative Oral Solution Lemon Flavor products manufactured by Vi-Jon, LLC and sold under various store brand names were recalled after testing identified the presence of the bacteria Gluconacetobacter liquefaciens.
Recalls & Warnings
June 16, 2004
Ads for Various Diet Pills and Topical Gels Don’t Cut the Fat, Says the FTC
On June 16, 2004, The Federal Trade Commission (FTC)charged a Utah-based company, five related corporations, and three individuals operating as a common enterprise with making numerous false and unsubstantiated claims for weight-loss and fat-loss gels and supplements.
Recalls & Warnings
September 14, 2023
FDA Warns CVS, Walgreens, Similasan & Others for Eye Drop Violations
On September 11, 2023, the FDA issued Warning Letters to the following eight sellers of homeopathic and other types of eye drops regarding the products noted in italics due to a variety of violations of FDA regulations, most notably that they were marked with claims suggesting that they could cure, ...
News Release
December 20, 2004
Testing of Alpha-lipoic acid supplements by ConsumerLab.com finds most meet label claims but one with only 15% of ingredient — Antioxidant of potential benefit in diabetes and other conditions
WHITE PLAINS, NY — December 20, 2004 — ConsumerLab.com announced today that one of 21 alpha-lipoic acid supplements recently tested contained only 15% of the alpha-lipoic acid that it claimed. Alpha-lipoic acid is an antioxidant naturally produced in the body.
News Release
July 07, 2004
ConsumerLab.com finds several herbal sleep supplements fail tests for quality — Results for 13 valerian products released today
WHITE PLAINS, NY — Wednesday, July 7, 2004 — ConsumerLab.com announced today that five valerian dietary supplements failed to pass recent testing due to low potency and/or contamination. Valerian, an herbal sleep aid, accounted for $47 million in sales in the U.S.
Recalls & Warnings
March 15, 2016
FDA Warns Sellers of Weight and Workout Supplements Containing Acacia Rigidula
On March 7, 2016, the FDA issued warning letters to five sellers of supplements which were labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
February 01, 2018
Seller of Supplements for Opiate Withdrawal Warned for Drug Claims
On January 11, 2018, the FDA sent warning letters to ten sellers of supplements promoted to treat opiate withdrawal following reviews of the companies' websites and social media which found statements and testimonials made about the products to be drug claims.
Recalls & Warnings
April 25, 2017
Beware of Products Which Promise to Treat or Cure Cancer, FDA Warns
On April 25, 2017, the FDA warned consumers to be aware of supplements and other products claiming to cure cancer.
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
March 02, 2021
FDA Warns Seller of Vitamin C, Silver Spray
On March 1, 2021, the FDA issued a warning letter to Ageless Global, LLC following a review of the company's websites for selling Immunoral, Immune Plus, MD Immune Support Spray, and MD CVK-365 Mouth Spray with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
February 22, 2021
FDA Warns Seller of Melatonin & Other Sleep Supplements
On February 18, 2021, the FDA issued a warning letter to SANA Group LLC following a review of the company's website, which found statements made about the company's products Sleep Sana Sleep Drops and Sleep Shots to be drug claims.
Recalls & Warnings
February 22, 2021
FDA Warns Sellers of St. John's Wort
On February 18, 2021, the FDA issued warning letters to two companies following reviews of the companies' websites, which found statements made about the companies' St. John's Wort products to be drug claims. These products include St.
Recalls & Warnings
February 02, 2021
Seller of Aloe Products Warned for Claiming to Treat Joint Stiffness
On January 22, 2021, the FDA issued a warning letter to American Global Health Group, LLC following a review of the company's website, which found statements made about the company's products AloeCure VeraFlex, AloeCure Advanced Formula Capsule, AloeCure Pure Aloe Vera ...
Recalls & Warnings
January 21, 2021
Federal Court Bars Fusion Health From Promoting Vitamin D for COVID-19
On January 8, 2021, the United States Department of Justice announced a permanent injunction has been entered, barring dietary supplement marketer Matthew Ryncarz and his companies Fusion Health and Vitality LLC dba Pharm Origins and Fusion Ionz LLC dba Pharm Origins from making claims that their ...
Recalls & Warnings
January 20, 2021
Seller of Omega-3 Warned for Making Claims to Treat Focus, Mood
On December 9, 2020, the FDA issued a warning letter to Bodyhealth.com, LLC following a review of the company's website, which found statements made about the company's products Healthy-Thin Energize, Body Detox (Oral Spray), and Omega 3 Health to be drug claims.
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Flu Immune
On December 21, 2020, the FDA issued a warning letter to Riverstone LLC for selling the products Flu Immune Drops, L-Lysine, Lysine Extra, and Monolaurin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Liposomal Vitamin C, Vitamin D & More
On December 21, 2020, the FDA issued a warning letter to Sparrow Health & Performance LLC for selling the products Organic Liposomal Vitamin C, Nanoemulsified D3K2 (also called Liquid Liposomal Vitamin D3 with K2 or Liposomal Vitamin D3) and Immune Support ...
Recalls & Warnings
September 05, 2020
Bio aaa Advance Hand Sanitizer Recalled
On September 3, 2020, AJR Trading LLC recalled one lot of bio aaa Advance Hand Sanitizer because different lots of the product may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
September 03, 2020
Red-E Male Enhancement Capsule Recalled
On September 1, 2020, The Protein Shoppe, LLC issued a recall of Red-E male enhancement capsules because FDA analysis found it to contain sildenafil.
Recalls & Warnings
September 15, 2020
FDA Warns Seller of Unapproved "COVID-19 test package"
On June 29, 2020, the FDA issued a warning letter to Pomegranate Consulting, LLC, Pomegranate Consulting, Ltd. dba Glorious One-Pot Meals for selling COVID-19 test package, an unapproved, adulterated, and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
September 11, 2020
M Hand Sanitizer Recalled
On September 9, 2020, Medek, LLC recalled M Hand Sanitizer Alcohol Antiseptic 80% 128 oz/3,785 mL because it may contain methanol, which is toxic when absorbed through the skin or ingested. The product may also have a sub-potent ethanol content, which leads to a lack of efficacy.
Recalls & Warnings
September 10, 2020
Hand Sanitizer Labeled as "Edible" Recalled
On September 3, 2020, CorgioMed LLC recalled one lot of Leafree Instant Hand Sanitizer Aloe Vera because they are labeled "edible alcohol." Hand sanitizers can be toxic if ingested, and can cause lack of coordination, slowed or slurred speech, drowsiness, coma, or death.
Recalls & Warnings
November 24, 2020
Recalled Vitamin D Contains Potentially Dangerous Ingredient
On November 22, 2020, Fusion Health and Vitality LLC recalled all 2020 lots of CORE Essential Nutrients and Immune Boost Sublingual Vitamin D3 because they are adulterated. CORE Essential Nutrients contains the unapproved food additive hordenine HCl.
Recalls & Warnings
August 10, 2020
Seller Indicted for Promoting Silver Product as Coronavirus Cure
On July 28, 2020, Utah resident Gordon Pedersen was indicted by a federal grand jury for posing as a medical doctor to sell an unapproved treatment for coronavirus (COVID-19).
Recalls & Warnings
July 07, 2020
Sellers of Essential Oils, Hand Sanitizers and More Warned for Coronavirus Claims
The FDA recently issued warning letters to five companies for selling products such as essential oils, homeopathic products, and Chinese herbal products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
November 10, 2020
FDA Warns Immusist for Drug Claims
On October 16, 2020, the FDA issued a warning letter to Immusist, LLC following a review of the company's website, which found statements made about the company's products IMMUSIST Original and IMMUSIST Natural to be drug claims.
Recalls & Warnings
November 10, 2020
Seller of Cholesterol-Lowering Supplements Warned for Drug Claims
On October 29, 2020, the FDA issued a warning letter to Natural Sprout Co.
Recalls & Warnings
October 30, 2020
Seller of Elderberry and Honey Products Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Beepothecary LLC for selling the products BEEHive Delight, BEEbread, and Elderberry, Honey & Propolis Syrup with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Simple Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 23, 2020, the FDA issued a warning letter to Peterson Research Laboratories LLC for selling the product Simple Silver with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
October 15, 2020
FDA Warns Five Sellers of Dangerous Cesium Salt Supplements
On October 9, the FDA issued warning letters to five companies for selling products containing cesium chloride. The FDA has previously warned consumers not to use dietary supplements containing cesium chloride or any other cesium salt.
Recalls & Warnings
December 15, 2020
FDA Warns Seller of Evening Primrose Oil and Beta Glucan
On December 2, 2020, the FDA issued a warning letter to Smoky Mountain Naturals, LLC following a review of the company's website, which found statements made about the company's products Evening Primrose Oil and Beta Glucan to be drug claims.
Recalls & Warnings
December 11, 2020
Seller of "Dr. Hotze's Immune Pak" Products Warned for COVID-19 Claims
Seller of "Dr. Hotze's Immune Pak" Products Warned for Coronavirus Claims
Recalls & Warnings
December 11, 2020
FDA Warns Seller of Curcumin and Cholesterol Supplements
On November 20, 2020, the FDA issued a warning letter to Natures Boost LLC following a review of the company's websites, which found statements made about the company's products Blood Boost Formula and Turmeric Curcumin to be drug claims.
Recalls & Warnings
December 08, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement Synovia
On December 1, 2020, the FTC announced it is mailing 13,221 checks totaling nearly $775,000 to consumers who bought Synovia, a supplement intended to treat joint pain and arthritis.
Recalls & Warnings
March 18, 2021
Hand Sanitizer Recalled Because Packaging Resembles Water Bottle
On March 17, 2021, PNHC, LLC d/b/a Heal the World recalled all lots of Heal the World hand sanitizer because the product is packaged in containers resembling water bottles.
Recalls & Warnings
December 15, 2020
Mushroom Supplement Seller Warned for Drug Claims
On December 4, 2020, the FDA issued a warning letter to Desert Alchemist LLC following a review of the company's website, social media, and Etsy.com store (www.etsy.
Recalls & Warnings
May 09, 2020
Federal Court Orders Seller to Stop Promoting Silver Product as Coronavirus Cure
On April 29, 2020, a federal court in Utah announced that it has obtained a temporary restraining order preventing Gordon Pedersen and his companies, My Doctor Suggests LLC and GP Silver LLC, from promoting fake treatments for coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 02, 2020
Seller of Botanical and CBD Oil Patches Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Santiste Labs LLC for promoting its transdermal patches containing botanical oils and/or CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 11, 2020
FDA Warns Sellers of CBD, Colloidal Silver & Natural Remedies Promoted to Treat Coronavirus
Between April 7 and April 9, 2020, the FDA issued warning letters to five companies for selling products such as CBD, colloidal silver, and natural treatments with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 28, 2020
Iron Supplements Recalled Due to Undeclared Allergen
Between July 23 and 24, 2020, two companies issued recalls of iron supplements because they may contain undeclared milk.
Recalls & Warnings
January 02, 2012
Manufacturing Violations for Multivitamin, Vitamin K and Other Supplements
On December 19, 2011, the U.S. FDA sent a Warning Letter to Milk Specialties Global regarding manufacturing violations. Affected products include PrimaForce Proliver Capsules, Dr. Mercola Vitamin K2, Active Women’s Multivitamin/Multimineral, Cremagnavol, Immune Support, and HPF Women’s Multi.
Recalls & Warnings
January 10, 2012
Acai Berry Pill Marketers to Pay $1.5 Million to Settle FTC Charges
On January 9, 2012, the U.S. Federal Trade Commission (FTC) announced that an operation that marketed acai berry supplements, "colon cleansers," and other products using allegedly fraudulent free trial offers and phony endorsements from Oprah Winfrey and Rachael Ray will pay $1.
Recalls & Warnings
November 02, 2009
FTC Charges Marketers with Baseless Weight-Loss Claims
On November 2, 2009 the U.S. Justice Department, at the Federal Trade Commission’s request, filed suit today in federal court in a case affecting consumers nationwide.
Recalls & Warnings
February 13, 2016
Cannabis Compound Not Permitted in Supplements, FDA Warns
On February 4, 2016, the FDA issued warning letters to eight companies selling products containing cannabidiol (CBD), a compound derived from cannabis (also known as marijuana). In the U.S., cannabidiol is not permitted to be sold as an ingredient in dietary supplements.
Recalls & Warnings
March 09, 2020
FDA Warns Sellers of Essential Oils, Colloidal Silver & Teas Promoted to Treat Coronavirus
On March 9, 2020, the FDA and FTC announced they have issued joint warning letters to seven companies for selling products such as essential oils, teas and colloidal silver with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
February 18, 2020
Seller of CoQ10, Resveratrol and More Warned for Manufacturing Violations
On February 5, 2020, the FDA issued a warning letter to R-Garden LLC, which found the company's Vitamin O, Gamma-Zyme, L.
Recalls & Warnings
December 21, 2019
Supplement Company Continues to Make False Claims About Its Products, Says FTC
The FTC has filed a motion of contempt against two supplement companies that, according to the motion, have continued to promote their products with false claims despite being barred from doing so by a previous court order.
Recalls & Warnings
December 01, 2020
Niagen Cannot Be Promoted to Prevent or Treat COVID-19, Warns FDA
On November 17, 2020, the FDA issued warning letters to the sellers of Niagen and Nadovim, supplements that contain different forms of niacin, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
Recalls & Warnings
July 06, 2020
FDA Warns Five More Hand Sanitizers May Contain Toxic Ingredient
Update: (7/14/20) The FDA has warned consumers not to use forty-six more hand sanitizers that may contain methanol.
Recalls & Warnings
August 17, 2020
SkinGuard24 Hand Sanitizer Recalled
On August 14, 2020, SG24 LLC recalled SkinGuard24 — All Day Hand Sanitizer because it is labeled as containing methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 20, 2020
FTC Warns 30 More Companies for Coronavirus Claims
On June 18, 2020, the FTC announced that it sent warning letters to 30 companies for selling products such as immune system boosters, colloidal silver, vitamin C, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 15, 2021
Toxic Hand Sanitizer Recalled
On May 11, 2021 Dibar Nutricional S. de R.L. de C.V. issued a recall of various lots of DIBAR Labs Hand Sanitizer and ProtectoRx Hand Sanitizer because they were confirmed to contain methanol.
News Release
May 28, 2004
ConsumerLab.com warns of deceptive campaign by manufacturer of children's vitamin
WHITE PLAINS, NY — FOR IMMEDIATE RELEASE — FRIDAY MAY 28, 2004 — Responding to a deceptive public relations campaign recently launched by vitamin manufacturer Northwest Natural Products, Inc., ConsumerLab.
Recalls & Warnings
November 21, 2024
Douglas Labs Stress-B-Plus Recalled
On October 7, 2024, Nestle Health Science (NHS U.S., LLC) issued a recall of two lots of Douglas Labs Stress-B-Plus because it contains incorrect information on the label.
News Release
April 27, 2004
New ConsumerLab.com report on St. John's wort highlights benefits and risks of herbal product for depression
NEW CONSUMERLAB.COM REPORT ON ST.
Recalls & Warnings
November 26, 2007
Encore Tabs Recalled -- Contain Prescription Drug
On November 21, 2007, Bodee LLC announced today that it is conducting a voluntary nationwide recall of all the company's supplement product sold under the name Encore Tabs.
Recalls & Warnings
April 09, 2006
Sellers of Children’s Weight-Loss Product Settle FTC Charges
On April 6, 2006, the Federal Trade Commission (FTC) announced that the marketers of Pedia Loss, a purported children’s weight-loss product, and Fabulously Feminine, a supposed female libido enhancement product, had agreed to settle Federal Trade Commission charges that they made false and ...
Recalls & Warnings
April 06, 2016
FDA Warns of Stimulant Methylsynephrine In Supplements
On March 31, 2016, the FDA issued warning letters to seven companies selling products containing methylsynephrine (also called p-hydroxyephedrine or Oxilofrine), a stimulant drug which is not permitted in dietary supplements in the U.S.
Recalls & Warnings
September 05, 2015
Powdered Caffeine Risky, FDA Warns
On August 27, 2015, the FDA issued a warning letter to five companies selling caffeine powder, warning that the powder "presents a significant or unreasonable risk of illness or injury under the conditions of use recommended or suggested in the labeling."
Recalls & Warnings
March 14, 2017
Court Shuts Down Supplement Manufacturer
On March 13, 2017, the U.S. District Court for Colorado ordered dietary supplement manufacturers and distributors EonNutra LLC, CDSM LLC and HABW LLC, and their owner, Michael Floren, to stop all operations until they come into compliance with federal regulations.
Recalls & Warnings
October 06, 2005
Deceptive Marketing of “Supreme Greens" -- Settlement with FTC
On October 6, 2005, the Federal Trade Commission (FTC) announced that three individuals and two companies have settled FTC charges over their roles in the deceptive marketing of Supreme Greens, an herbal supplement.
Recalls & Warnings
April 09, 2019
Dangerous Levels of Lead and Nickel Found in Kratom Products
On April 3, 2019, the FDA released the results of its tests that found dangerous levels of lead and nickel in certain kratom products. The agency tested 30 products sold on websites such as krakenkratom.com and by retailers such as Gaia Ethnobotanicals, Sunstone Organics LLC and Happy Hippo LLC.
Recalls & Warnings
December 02, 2020
FDA Warns Sellers of Unapproved COVID-19 Tests, CBD Products
On November 23 and November 30, 2020, the FDA issued warning letters to Industry Lab Diagnostic Partners and Avazo-Healthcare, LLC, respectively, for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
Recalls & Warnings
October 09, 2023
Organifi Settles Charges of Unsupported Claims
Organifi, LLC has agreed to pay $150,000 in civil penalties and $50,000 in investigative costs as restitution to settle charges that the company made unsupported claims that its Gold, Pure, Green Juice, and Red Juice products could balance hormone and cortisol levels, regulate the ...
Recalls & Warnings
November 06, 2023
FDA Warns Consumers Not to Use Dr. Ergin’s SugarMD Advanced Glucose Support, Found to Contain Prescription Drugs
On November 3, 2023, the FDA warned consumers not to purchase or use Dr. Ergin’s SugarMD Advanced Glucose Support products after FDA laboratory analysis found the supplement to contain glyburide and metformin.
Recalls & Warnings
November 09, 2023
FDA Warns Biorica International for Cholesterol, Kidney & Cancer Claims
On August 4, 2023, the FDA issued a Warning Letter to Biorica International Corp. following review of the company’s websites and social media, which found statements about the company’s Plaquex Oral Supplements to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
April 27, 2023
FTC Returns over $1.1 Million to Consumers for RevMountain Teeth Whitening Products
On April 25, 2023, the FTC announced it will be returning over $1.
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
News Release
April 07, 2004
ConsumerLab.com finds some zinc supplements low in ingredient and many don't instruct on proper use — Consumers urged to learn more about zinc when using supplements
WHITE PLAINS, NY — April 7, 2004 (updated May 4, 2004) — ConsumerLab.com announced today that two of nine zinc pill or liquid supplements that it recently tested contained only 73% to 85% of the claimed amounts.
News Release
March 18, 2004
Many echinacea supplements don't meet quality standards according to ConsumerLab.com — Test results of cold-reducing herbal supplements released
WHITE PLAINS, NY — March 18, 2004 — ConsumerLab.com announced today that five echinacea supplements failed to pass recent independent testing. Lower than claimed amounts of key compounds such as echinacoside and phenols were the most common problem.
News Release
January 09, 2004
USANA products pass ConsumerLab.com screening for substances banned from Olympics
WHITE PLAINS, NY — January 9, 2004 — ConsumerLab.com announced today that six additional products have passed its Athletic Banned Substances Screening Program. ConsumerLab.com tested the products at the request of USANA, a supplement manufacturer. ConsumerLab.
News Release
December 08, 2003
ConsumerLab.com dispels myths about HGH human growth hormone supplements sold widely on Internet
WHITE PLAINS, NY — December 8, 2003 — ConsumerLab.com has released a review of the scientific evidence behind human growth hormone (hGH or HGH) supplements.
News Release
November 02, 2003
Key ingredient missing in some arthritis supplements for people and pets according to ConsumerLab.com — Review of glucosamine, chondroitin and MSM supplements published online today
WHITE PLAINS, NY — November 2, 2003 — ConsumerLab.com today announced test results for supplements used in treating osteoarthritis that contain glucosamine, chondroitin, and/or MSM.
News Release
January 21, 2003
Acidophilus" and other probiotic supplements gain in popularity but live bacteria missing in many — ConsumerLab.com releases review online today
WHITE PLAINS, NY — January 21, 2003 — ConsumerLab.
News Release
December 30, 2002
Top quality melatonin products identified by ConsumerLab.com — Hormone supplement used to treat sleep disturbances due to jet travel and other causes
WHITE PLAINS, NY — December 30, 2002 — In its latest Product Review, ConsumerLab.com found that 16 of 18 melatonin dietary supplements met their label claims and were free of lead contamination.
News Release
December 10, 2002
Lupus patients cautioned about hormone supplement — ConsumerLab.com releases DHEA testing results online today
WHITE PLAINS, NY — December 10, 2002 — In its latest Product Review, ConsumerLab.com found that 3 of the 17 DHEA (dehydroepiandrosterone) supplement products it tested contained less than their claimed amounts of this hormone — one having less than one-fifth of what it claimed.
News Release
October 29, 2002
Enormous variation found in strength of garlic supplements — most popular herb in U.S. — ConsumerLab.com releases results online today
WHITE PLAINS, NY — October 29, 2002 — ConsumerLab.com's Product Review of Garlic Supplements found strength to vary by as much as 1500% across products. Strength was based on each product's ability to generate allicin, a chemical associated with the efficacy of non-aged garlic.
News Release
September 24, 2002
Problems found with most sexual enhancement supplements evaluated by ConsumerLab.com — Only 9 of 22 products pass independent review
WHITE PLAINS, NY — September 24, 2002 — ConsumerLab.com announced today that nearly 60% of products failed to pass its independent Product Review of Sexual Enhancement Supplements. Sexual dysfunction is estimated to affect 43% of women and 31% of men in the U.S.
News Release
August 20, 2002
ConsumerLab.com announces screening of supplements for banned substances for the United States Olympic Committee — First products to pass now listed on ConsumerLab.com web site
WHITE PLAINS, NY — August 20, 2002 — The United States Olympic Committee (USOC) and ConsumerLab.com (CL) announced today that nine products have passed ConsumerLab.com's recently launched Athletic Banned Substances Screening Program. ConsumerLab.
News Release
August 07, 2002
ConsumerLab.com finds some spoilage and inaccuracy among omega-3 and 6 supplements — List released of evening primrose, flaxseed and other GLA/ALA products that passed testing
WHITE PLAINS, NY — August 7, 2002 — ConsumerLab.com, an independent evaluator of health and nutrition products, released results today of its Product Review of Black Currant, Borage, Evening Primrose, and Flaxseed Oils: Sources of ALA and GLA (Omega-3 and -6 Fatty Acids).
News Release
July 10, 2002
Pharmavite dietary supplements receive ConsumerLab.com approval — Vitamin E, SAM-e, St. John's Wort, Ginkgo and others merit approved quality products ranking
WHITE PLAINS, NY — July 10, 2002 — ConsumerLab.
News Release
April 16, 2002
ConsumerLab.com finds "Breast Enhancement" pills lack evidence of efficacy
WHITE PLAINS, NY — April 16, 2002 — ConsumerLab.com announced today that it found no hard evidence to suggest that current dietary supplements marketed for breast enhancement are effective.
News Release
March 28, 2000
Only half of SAM-e supplements found to contain proper ingredients and labeling in ConsumerLab.com testing; Product Review of popular anti-depressant published online today.
WHITE PLAINS, NY, March 28, 2000 — Among 13 brands of SAM-e dietary supplements (used primarily in treating depression and osteoarthritis) only seven were found to contain labeled amounts of SAM-e (S-adenosyl-methionine). These results were reported today by ConsumerLab.
News Release
March 07, 2000
ConsumerLab.com finds many arthritis supplements lacking labeled ingredients; Glucosamine and Chondroitin product review published online today.
WHITE PLAINS, NY, March 7, 2000 — Among 25 major brands of supplements containing glucosamine and/or chondroitin used for treating osteoarthritis, nearly one-third were found not to contain all labeled ingredients. These results were reported today by ConsumerLab.
News Release
August 05, 1999
ConsumerLab.com initiates independent testing of herbal products and supplements. Certification Seal announced.
WHITE PLAINS, NY, August 5, 1999 — ConsumerLab.com, LLC announced that it has begun a large-scale program of independent testing of herbal products and dietary supplements. .
Recalls & Warnings
January 31, 2012
FTC Stops Fake News Sites About Acai Berry Products
As published on the U.S. Federal Trade Commission's (FTC) website on January 25, 2011, six online marketers settled with the FTC to stop posting fake news on websites intended to promote acai berry supplements and other weight-loss products.
Recalls & Warnings
April 26, 2015
FDA Identifies More Products Listing Synthetic Amphetamine
On April 22, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called beta- methylphenylethylamine (BMPEA) on product labels.
Recalls & Warnings
December 27, 2012
Two Supplement Companies Warned For Manufacturing Violations
Sterling USA Neutraceutical Lab, LLC -- On November 16, 2012, the FDA issued a warning letter to Sterling USA Neutraceutical Lab, LLC following a facility inspection which found the company’s dietary supplements to be adulterated because they were have been prepared, packed, or held under ...
Recalls & Warnings
June 20, 2017
Beware of Bodybuilding Supplements, FDA Warns
On June 20, 2017, the FDA warned consumers to beware of bodybuilding products because a number of these products have been found to contain steroids or steroid-like substances. Such products could cause serious and even life-threatening health risks, including:
Recalls & Warnings
December 05, 2015
FDA Warns Companies Selling Products Containing Picamilon
On November 30, 2015, the FDA issued warning letters to five supplement companies selling products containing picamilon, which is not a lawful dietary supplement ingredient in the U.S.
Recalls & Warnings
April 03, 2020
FDA Warns Seller of FullerLifeC60 for Coronavirus Claims
On March 30, 2020, the FDA issued a warning letter to FullerLifeC60, LLC, for selling a product with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
January 12, 2023
FDA Warns Two Sellers Promoting CBD to Treat COVID-19
On January 10, 2023, the FDA issued warning letters to two companies following a review that found statements made on the companies’ websites to be drug claims because they promoted the cannabidiol (CBD) products to prevent or treat COVID-19.
Recalls & Warnings
July 06, 2022
FDA Warns Kratom Companies for Arthritis, Pain Relief Claims & More
On January 30, 2022, the FDA issued warning letters to four sellers of kratom products because they were promoted to treat pain relief, opioid withdrawal, blood sugar control, anxiety, and to treat rheumatoid arthritis.
Recalls & Warnings
June 17, 2022
Built Brand Protein Bar Recalled Due to E. Coli Concern
On June 10, 2022, Built Brands, LLC. Issued a recall of 4,146 Banana Cream Pie Puffs protein bars after routine testing discovered potential contamination with E. coli (Escherichia coli).
Recalls & Warnings
June 16, 2004
FTC Challenges Ads for Kids’ Weight Loss Pill and Female Sexuality Supplement
On June 16, 2004 The Federal Trade Commission announced that it had charged three Florida-based companies and their principals with making false and unsubstantiated claims in connection with the advertising for “Pedia Loss,” a purported children’s weight loss product.
Recalls & Warnings
December 06, 2011
FTC Stops Operator of Fake News Sites Offering Acai and Colon Cleanse Products
On December 1, 2011, The Federal Trade Commission announced that it has filed a complaint jointly with the State of Connecticut, seeking to permanently stop a Connecticut-based operation that allegedly used fake news websites to promote their products, made deceptive weight-loss claims, and told ...
Recalls & Warnings
February 01, 2022
Complaint Submitted to FTC for doTerra Essential Oil COVID Claims
On January 28, 2022, the website truthinadvertising.org submitted a complaint to the Federal Trade Commission (FTC) against doTerra International, LLC. following a series of Zoom calls from the company titled "Protocols for the Current Climate.
Recalls & Warnings
May 24, 2021
U.S. Marshals Seize $1.3 Million Worth of Kratom
U.S. Marshals have seized approximately $1.3 million worth of bulk kratom and kratom supplements manufactured by Atofil, LLC, a subsidiary of Premier Manufacturing Products, according to the FDA.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
July 19, 2011
Recall of Enhancement Supplement for Men - Contains Drug Compounds
On July 13, 2011, Global Wellness, LLC announced an expanded voluntary nationwide recall of Via Extreme Ultimate Sexual Enhancer Dietary Supplement for Men. The product was distributed throughout the U.S. Puerto Rico, Barbados, and Canada to internet and retail consumers.
Recalls & Warnings
January 18, 2013
FTC Upholds Ruling, POM Wonderful Health Claims Were Deceptive
On January 16, 2013, the Federal Trade Commission (FTC) announced it will uphold a judge’s ruling that makers of POM Wonderful 100% Pomegranate Juice and POMx supplements made deceptive and unsubstantiated claims that the products could treat, prevent, or reduce the risk of heart disease, prostate ...
Recalls & Warnings
April 29, 2013
Recall: Multivitamin Containing Undeclared Milk
On April 26, 2013, Saratoga Therapeutics, LLC issued a recall of ebA Multivitamin Supplement because it may contain undeclared milk. The label describes the supplement as free of milk components.
Recalls & Warnings
February 20, 2013
Recall: Arthritis and Muscle Pain Supplement Contains Prescription Drugs
On February 15, 2013, Reumofan Plus USA, LLC and Reumofan USA, LLC announced a recall of Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain, because it was found to contain the active pharmaceutical ingredients methocarbamol, dexamethasone, and diclofenac.
Recalls & Warnings
January 13, 2015
Seller of Speech Disorder Supplement Agrees to Settle FTC Charges of Deceptive Claims
NourishLife, LLC, seller of supplements promoted for treating children's speech disorders, has agreed to pay $200,000 in order to settle FTC charges that claims made on the company's website and in brochures were deceptive and not supported by science.
Recalls & Warnings
May 20, 2014
FTC Charges Seller of Green Coffee Bean with False Weight Loss Claims, Fake Websites
On May 15, 2014, the Federal Trade Commission (FTC) filed a lawsuit against the sellers of Pure Green Coffee for making unsubstantiated weight loss claims and deceiving consumers with fake "news" websites.
Recalls & Warnings
January 12, 2016
Twenty-four Male Enhancement Supplements Recalled
On January 9, 2016, R Thomas Marketing LLC. in conjuction with Just Enhance LLC, issued a recall of the following sexual enhancement supplements because they were found to contain sildenafil:
Recalls & Warnings
April 28, 2017
Marketers of Weight Loss System Agree to Settle FTC Charges of Deceptive Claims
On April 21, 2017, the FTC announced the marketers of the NutriMost Ultimate Fat Loss System (NutriMost, LLC and NutriMost Doctors, LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
October 11, 2016
Seller of Glucosamine and Chondroitin Settles FTC Charges of False Advertising
On October 5, 2016, the FTC announced the sellers of the liquid glucosamine and chondroitin supplement Supple (Supple LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
September 06, 2016
Seller on Noni Juice Warned for Manufacturing Violations, Drug Claims
On August 26, 2016, the FDA issued a warning letter to Healing Noni Co. L.L.C.
Recalls & Warnings
January 24, 2003
FTC Challenges Weight-loss Claims for Slim Down Solution
The Federal Trade Commission today charged Slim Down Solution, LLC, Maderia Management, Inc., and several related companies and individuals with using false and unsubstantiated claims in the marketing and advertising of "Slim Down Solution" - a purported weight-loss product.
Recalls & Warnings
December 08, 2011
HCG Diet Products Don't Work and Are Illegal Says FDA
On December 6, 2011, the U.S. FDA warned consumers to avoid homeopathic HCG weight-loss diet products because they don't work, often make unsupported claims, and are illegal for sale. In addition, some of these products direct user to follow a potentially dangerous diet.
Recalls & Warnings
September 24, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement
On September 24, 2020, the FTC announced it is mailing 4,782 checks totaling more than $76,000 to consumers who bought Isoprex, a supplement marketed with deceptive claims that it could treat or cure joint pain, muscle pain, headaches, and arthritis.
Recalls & Warnings
August 13, 2020
FDA Warns Three More Companies Selling Unapproved COVID-19 Tests
Between July 23 and 24, the FDA issued warning letters to three companies for marketing unapproved, adulterated or misbranded antibody tests for coronavirus (COVID-19).
Recalls & Warnings
August 25, 2020
Seller of CBD and NAC Warned for Coronavirus Claims
On August 19, 2020, the FDA issued a warning letter to Living Senior, LLC for selling CBD and NAC products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 18, 2020
Seller of Immune Shot Criminally Charged With Making Coronavirus Claims
On August 10, 2020, prosecutors in Georgia charged Matthew Ryncarz and his company Fusion Health and Vitality, LLC d/b/a/ Pharm Origins with selling the misbranded product Immune Shot.
Recalls & Warnings
June 23, 2020
KBMO Diagnostics Warned for Unapproved COVID-19 Test
On June 17, the FDA issued a warning letter to KBMO Diagnostics, LLC for selling the product COVID-19 Fingerstick Test Kit, an unapproved, adulterated and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
July 10, 2020
Seller of Hand Sanitizer "Alternative" Warned for Coronavirus Claims
On July 7, 2020, the FDA issued a warning letter to Ionogen, LLC for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 29, 2020
Herbacil Antiseptic Hand Sanitizer Recalled
On July 27, 2020, Broncolin S.A. de C.V. recalled Herbacil Antiseptic Hand Sanitizer 70% Alcohol because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
April 03, 2020
Gaia's Whole Healing Essentials Warned for Colloidal Silver Coronavirus Claims
On April 1, 2020, the FDA issued a warning letter to Gaia's Whole Healing Essentials, LLC, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 14, 2020
Teami Tea Settles Charges of Unproven Claims and Deceptive Celebrity Endorsements
On March 6, 2020, the FTC announced that Teami, LLC, a marketer of tea and skin care products, agreed to settle charges that it was selling products with unproven claims and misleading celebrity endorsements.
Recalls & Warnings
April 17, 2020
Arthritis Supplement Recalled for Salmonella Risk
On April 13, 2020, Arthri-D, LLC issued a recall of one lot of Arthri-D arthritis supplement because recent testing found it has the potential to be contaminated with Salmonella.
Recalls & Warnings
April 25, 2020
Injectable Curcumin, CBD Are Not Safe, Warns FDA
On April 9, 2020, the FDA issued a warning letter to BIOTA Biosciences LLC following a review of the company's website, which found statements made about some of the company's, products, including Canabidiol (CBD) Complex, Cannabidiol+Curcumin, and Curcumin Complex to be ...
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
May 12, 2020
FDA Warns Seller of "COVID-19 Cough Syrup"
On May 8, 2020, the FDA issued a warning letter to Seanjari Preeti Womb Healing, L.L.C. for selling its product COVID-19 Cough Syrup with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
Recalls & Warnings
June 16, 2020
NutraCap Labs Warned for Unsafe Ingredients and Manufacturing Violations
On May 21, 2020, the FDA issued a warning letter to NutraCap Labs LLC, which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
June 16, 2020
Seller of Silver, Arginine, and More Warned for Manufacturing Violations
On June 1, 2020, the FDA issued a warning letter to Morningstar Minerals LLC, which found the company's products, including Silver Boost, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
June 05, 2020
Seller of Silver and Vitamin C Lozenges & More Warned for Coronavirus Claims
On June 1, 2020, the FDA issued a warning letter to Dr.
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
May 23, 2020
BIOTA Biosciences Recalls Injectable CBD and Curcumin Products
On May 20, 2020, BIOTA Biosciences LLC issued a recall of five lots of three injectable products because they were marketed without FDA approval.
Recalls & Warnings
May 28, 2020
FDA Warns Sellers of CBD, Colloidal Silver, Essential Oils, and More Promoted to Treat Coronavirus
On May 26, 2020, the FDA issued warning letters to four companies for selling products such as CBD, essential oils, colloidal silver, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 26, 2020
Seller of Vitamin and CBD "NoronaPak" Warned for Coronavirus Claims
On May 21, 2020, the FDA issued a warning letter to Apollo Holding LLC for promoting a kit containing vitamins and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 20, 2021
Chiropractor Who Claimed Vitamin D and Zinc Work Better Than COVID Vaccines Charged by FTC
On April 15, 2021, the FTC charged St.
Recalls & Warnings
March 04, 2022
FTC, FDA, and DOJ Take Joint Action Against Herbal Tea Companies for COVID Claims
On March 3, 2022, the FDA, FTC, and DOJ sued a New York-based marketer of herbal tea, in attempts to permanently block deceptive ads that claim Earth Tea is clinically proven to treat, cure, and prevent COVID-19.
Recalls & Warnings
December 07, 2011
Maker of PowerGum and Colon Cleanser Warned of Violations by FDA
The U.S. FDA published a Warning Letter (dated 12/1/11) to DreamLife, LLC regarding violations of Current Good Manufacturing Practices at its manufacturing facility in Louisana and labeling violations on its PowerGum and Extreme Colon Cleanser products.
Recalls & Warnings
October 05, 2011
FDA Warns Nature's Rite of Promoting 8 Supplements as Drugs
The FDA published a Warning Letter dated September 19, 2011 to Nature's Rite, LLC stating that the firm’s online promotion of its various supplements for the treatment or prevention of various diseases cause these products to be considered unapproved drugs.
Recalls & Warnings
November 21, 2012
Maker of Probiotic and Sports Drinks Warned For Manufacturing Violations
On November 6, 2012, the FDA issued a warning letter to CocoKefir, LLC stating that the company's Tula's CocoKefir, Tula's Citrus CocoKefir, and Tula's Apple Cinnamon CocoKefir have been prepared, packed or held under conditions which violate Current Good Manufacturing Practices.
Recalls & Warnings
July 29, 2008
Recall of Sexual Enhancement Supplements Containing Drug
On July 28, 2008 the U.S. FDA announced that Jack Distribution, LLC and G & N works, Inc. are conducting a voluntary nationwide recall of all lot numbers of the company's supplement products sold under the brand names Rize 2 The Occasion and Rose 4 Her.
Recalls & Warnings
June 21, 2005
FTC Settles Claims with Marketers of FiberThin and Propolene
On June 20, 2005, the Federal Trade Commission (FTC) announced that the marketers of the dietary supplements FiberThin and Propolene have settled FTC charges that their misleading weight-loss claims violated federal laws.
Recalls & Warnings
June 07, 2013
Seller of Red Rice Yeast With CoQ10, Respiratory Supplement And More Warned For Manufacturing Violations and Drug Claims
On February 25, 2013, the FDA issued a warning letter to Altasource, LLC, dba Meta Labs LLC, following a facility inspection which found the company's dietary supplements, including Respiratory Response, African Mango, Coffee Black Salve, Meta-Cell, Conjugated Linoleic Acid and Red Yeast Rice with ...
Recalls & Warnings
April 17, 2018
Sexual Enhancement Supplement Recalled
On April 16, 2018, Epic Products, LLC, recalled its sexual enhancement supplement Euphoric because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
August 07, 2018
FDA Warns Seller of Noni Juice Products
On July 18, 2018, the FDA issued a warning letter to Hawaiian Organic Noni, LLC, following a facility inspection which found statements made on the labels of certain products, including Hawaiian Organic Noni Fruit Leather, Hawaiian Organic Noni Banana Fruit Leather, Hawaiian Organic Noni ...
Recalls & Warnings
May 07, 2019
"The Beast" Supplement for Men Recalled
On May 7, 2019, STIFF BOY LLC issued a recall of The Beast, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
December 05, 2019
Joint Supplement Synovia Was Promoted With Phony Testimonials, Says FTC
A.S. Research, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers with false claims that their dietary supplement Synovia could treat arthritis and alleviate joint pain.
Recalls & Warnings
April 12, 2019
Sexual Enhancement Supplement for Men Recalled
On April 9, 2019, SD Import, LLC issued a recall of Aphrodisiac, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
November 02, 2019
Brain Supplement Claims to Treat Brain Injuries, Alzheimer's Are Deceptive, Says FTC
On November 1, 2019 the FTC filed a lawsuit against Neora LLC (formerly Nerium International) for making false claims that the supplement Neora EHT (formerly called Nerium EHT) can treat concussions and chronic traumatic encephalopathy caused by repetitive brain trauma, Alzheimer's ...
Recalls & Warnings
October 22, 2019
Company Marketing CBD for Use in Infants and Children Gets Strong Warning
On October 10, 2019, the FDA and FTC issued a joint warning letter to Rooted Apothecary LLC for "illegally selling unapproved products containing cannabidiol (CBD) online with unsubstantiated claims" including that the products could treat teething pain and ear aches in infants, autism, ...
Recalls & Warnings
October 22, 2019
Seller of TrueAloe and AloeCran Settles Charges of Making False Claims
NatureCity, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers by making false claims about their TrueAloe and AloeCran products.
Recalls & Warnings
July 12, 2019
PROBAR Meal Bars Recalled
On July 11, 2019, PROBAR LLC issued a recall of certain flavors and lots of its Meal® bars because they may contain undeclared milk and soy allergens. There has been one report of an allergic reaction to-date.
Recalls & Warnings
February 04, 2020
Seller of Digestion Supplements Warned for Manufacturing Violations
On January 10, 2020, the FDA issued a warning letter to Marco Pharma International LLC, which found the company's Absinthium Herbal Liquid Extract 100 ml (Absinthium), promoted for "digestive support", and other products, including S21 Multi Somaplex 100 ml (Multi Somaplex), ...
Recalls & Warnings
January 28, 2020
Weight Supplement Contains Hidden Drug
On January 13, 2020, the FDA issued a warning letter to Wave Miami, LLC because the company's weight loss supplement, Lipro Dietary Capsule was found to contain the prescription medication tadalafil.
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
February 09, 2021
Three More Hand Sanitizers Contain Toxic Ingredient
Between February 3 and 5, 2021, the FDA issued warning letters to three sellers of hand sanitizers because laboratory tests showed that their products contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
November 07, 2005
FTC Stops Bogus Ads for "Bio Trim" and Other Weight-loss Products
On November 7, 2005, the Federal Trade Commission (FTC) announced that under the terms of a consent agreement that it has approved, Tustin, California based Natural Products, LLC, All Natural 4 U, LLC and their owner, Ana M.
Recalls & Warnings
November 01, 2017
Hemp Oil and Cannabidiol (CBD) Marketers Warned by FDA Over Claims
On November 1, 2017, the FDA announced it issued warning letters to four companies promoting products containing cannabidiol (CBD), a compound derived from cannabis (also known as marijuana), for the treatment of cancer, as well as other diseases, such as Alzheimer's disease. In the U.
Recalls & Warnings
June 25, 2019
Kratom Is Dangerous and Not Proven to Treat Addiction, FDA Warns Marketers
On June 25, 2019, the FDA issued warning letter to two marketers and distributors of kratom products, listed below, for illegally selling unapproved, misbranded kratom-containing drug products with unproven claims about their ability to treat or cure opioid addiction and withdrawal symptoms.
Recalls & Warnings
June 04, 2004
FTC Charges Marketers of Two Supplements with False Claims to Cure Range of Diseases
On June 3, 2004 the Federal Trade Commission (FTC) charged marketers of two dietary supplements with falsely claiming that their products can prevent and cure cancer and other diseases. According to the FTC’s complaint, Boston-area marketers Direct Marketing Concepts, Inc. (DMC), ITV Direct, Inc.
Recalls & Warnings
August 18, 2006
Restitution Program for Purchases of Lane Labs' Products
On August 17, 2006 the Food and Drug Administration (FDA) announced that it was notifying consumers of a restitution (refund) program for purchasers of three of Lane Labs-USA, Inc.'s products. The products are BeneFin, MGN-3 and SkinAnswer.
Recalls & Warnings
October 05, 2007
FTC Charges Progesterone Cream Sellers with Making Unsubstantiated Claims
On October 5, 2007 the Federal Trade Commission (FTC) announced complaints against seven online sellers of alternative hormone replacement therapy (HRT) products, alleging that they made health claims for their natural progesterone creams without supporting scientific evidence.
Recalls & Warnings
October 06, 2006
FDA Warns Mangosteen Juice Maker of Drug Promotions
On September 20, 2006, the U.S. Food and Drug Administration (FDA) sent a Warning Letter to the makers of Xango, a mangosteen juice product. The letter advised that distributors of the product were using brochures making health claims restricted to the promotion of drugs.
Recalls & Warnings
November 14, 2012
Maker of Vitamin C, Aloe and Sexual Enhancement Supplements Warned for Drug Claims and Misbranding
On October 22, 2012, the FDA issued a warning letter to Health Breakthroughs International, LLC after a review of the company's product labels and website found the dietary supplements Amazing C, MPS-Gold 100 and Power Herbal Formula to be promoted as drugs.
Recalls & Warnings
July 24, 2012
Baby Supplement Recalled Due To Salmonella Risk
On July 20, 2012, Wellements LLC issued a voluntary recall of Baby Move Prune Concentrate because testing of retained raw materials used in the product found one ingredient to be positive for Salmonella.
Recalls & Warnings
June 13, 2012
Lack of Quality Control and Unapproved Claims by Supplement Seller
On May 14, 2012, the U.S. FDA sent a Warning Letter to Caribe Natural, LLC, distributor of GERMA brand dietary supplements, regarding violation of various FDA requirements.
Recalls & Warnings
May 16, 2012
Reaction Nutrition Warned by FDA of Violations in Manufacturing and Promotion of Several Supplements
On May 8, 2012, the U.S. FDA warned Reaction Nutrition, L.L.C. of Carnegie, PA of violations of Current Good Manufacturing Practices for dietary supplements and of illegally promoting a supplement as a drug.
Recalls & Warnings
May 07, 2012
FDA Warns Doctor Promoting Own Supplements Online as Treatments
On April 18, 2012 the U.S. Food and Drug Administration (FDA) sent a Warning Letter to Jacob Teitelbaum, M.D. of Fatigued to Fantastic, LLC regarding the promotion that company's products on its website www.endfatigue.com.
Recalls & Warnings
September 08, 2011
FDA Warns Several Supplement Manufacturers Not Following Good Manufacturing Practices
In recent weeks, the U.S. FDA has sent warning letters to several supplement manufacturers regarding deficiencies identified during audits of their facilities and not adequately corrected. Use the links below to access the Warning Letters on the FDA website.
Recalls & Warnings
January 04, 2012
Seller of "HCG Drops" for Weight Loss Warned by FDA and FTC of Violations
On December 21, 2011, the U.S. FDA and FTC sent a joint Warning Letter to hCG Drops LLC regarding its marketing of Slim Diet Drops. According to the letter, the product is considered an unapproved drug because it is promoted for drug-like uses on the company's website, including the following:
Recalls & Warnings
May 24, 2013
Brewable Tea and Tea Supplement Company Warned For Manufacturing Violations and Drug Claims
On April 2, 2013, the FDA issued a warning letter to Natures Health Options, LLC, following an investigation which found the company's Charantea Bitter Melon Ampalaya, which is distributed as a dietary supplement in capsule form, and as a tea, to be adulterated because it was prepared, packed, or ...
Recalls & Warnings
May 24, 2013
Maker of Neuropathic Pain Product Warned For Drug Claims, Misbranding
On April 11, 2013, the FDA issued a warning letter to Realm Labs, LLC, following a review of the company’s websites which found statements made about its NeuRemedy products to be drug claims.
Recalls & Warnings
August 08, 2013
Sleep Supplement Recalled Due To Undeclared Drugs
On August 7, 2013, Health and Beyond LLC issued a voluntary recall of dietary supplement Tranquility because it was found to contain traces of undeclared drugs, Doxepin and Chlorpromazine.
Recalls & Warnings
August 06, 2013
Maker of Greens and Whole Foods Supplements Warned For Drug Claims, Manufacturing Violations
On July 19, 2013, the FDA issued a warning to ProNatural Nutrition, LLC, following a facility inspection which found that statements made on product labels, including "Healing to cells and tissues," and "Non-toxic antibiotic" to be drug claims.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
Recalls & Warnings
August 28, 2013
Tea and Papaya Supplement Company Warned For Drug Claims
On August 12, 2013, the FDA issued a warning letter to Herbal Papaya, LLC, following a review of the company's website and Facebook page which found statements made about Papaya Seed Extract Capsules, 100% Papaya Leaf (Paw Paw Twig) and Papaya Leaf with Rooibos Tea to be drug claims.
Recalls & Warnings
November 21, 2012
Distributor Warns of Counterfeit Liquid Energy Shots
On November 19, 2012, Living Essentials LLC announced the company discovered and halted the production of counterfeit versions of 5-hour ENERGY® liquid energy shots. The company filed suits against the counterfeiters in U.S.
Recalls & Warnings
February 13, 2013
Herbal Supplement Maker Warned For Manufacturing Violations, Misbranding
On February 6, 2013, the FDA issued a warning letter to Genesis Herb Company LLC, following a facility inspection which found the company's Seizure, Relax, Formula ARTH, Disintegrate, Gout, Plantain, and Colloidal Silver dietary supplements to be adulterated because they were prepared, packed, or ...
Recalls & Warnings
August 03, 2017
AMPT "Sexual Enhancement" Coffee Recalled
On August 1, 2017, AMPT Life, LLC, recalled all lots of AMPT Coffee after FDA testing found sildenafil and tadalafil.
Recalls & Warnings
July 18, 2017
Herbal "Sexual Enhancement Coffee" Recalled
On July 13, 2017, Bestherbs Coffee LLC recalled their product New Kopi Jantan Tradisional Natural Herbs Coffee after FDA testing found desmethyl cabodenafil.
Recalls & Warnings
August 05, 2016
Seller of Fertilix Warned By FDA
On July 22, 2016, the FDA issued a warning letter to CellOxess LLC, following a review of the company's websites, social media pages and marketing literature which found statements made about Fertilix Preconceptual, Fertilix Low Dose and Fertilix Max to be drug claims.
Recalls & Warnings
October 29, 2016
Steroid "Alternatives" Not Permitted In Dietary Supplements, FDA Warns
On September 27, 2016, the FDA issued a warning letter to Proprietary Wellness, L.L.C.
Recalls & Warnings
November 01, 2017
FDA Warns Sellers of Bodybuilding Supplements Containing Steroid-Like Substances
On October 23, 2017, the FDA issued warning letters to three supplement distributors because several of their bodybuilding products contain selective androgen receptor modulators (SARMs).
Recalls & Warnings
September 08, 2017
Probiotic for Infants Recalled Due to Potential Choking Hazard
On September 7, 2017, Garden of Life, LLC, issued a recall of Baby Organic Liquid, a liquid probiotic supplement for infants, because the product, as labeled, includes directions for use that may be misinterpreted and could pose a choking hazard due to the thickness of the liquid.
Recalls & Warnings
September 16, 2015
Herbal Extracts Recalled
On September 15, 2015, Iowa Select Herbs, LLC issued a recall of numerous herbal exacts following a permanent injunction which required the company to stop selling supplements.
Recalls & Warnings
September 02, 2015
Antioxidant Supplement Recalled
On August 28, 2015, VRVK Nutraceuticals, LLC, DBA Dr. Venessa's Formulas, issued a voluntary recall of Ultimate Antioxidant Tablets because they may contain the undeclared allergens crustacean shellfish and milk.
Recalls & Warnings
November 21, 2015
FTC Charges Maker of Supplement for Opiate Addiction with Deceptive Claims
On November 16, 2015, the FTC filed a lawsuit against Sunrise Nutraceuticals, LLC, charging that the company made deceptive claims its product, Elimidrol, can alleviate opiate withdrawal symptoms and increase a user's likelihood of overcoming opiate addiction.
Recalls & Warnings
May 02, 2016
Liquid Multi Recalled Due to Allergen Risk
On April 26, 2016, Schaffner Distributing Pronutri LLC. issued a recall of Re-VITA-lize Whole Food Liquid Vitamin (in tropical orange flavor) because it contains undeclared soy lecithin.
Recalls & Warnings
May 17, 2016
Sexual Enhancement Supplement Contains Undeclared Drugs
On May 6, 2016, the FDA issued a warning letter to Economax, LLC because the company's sexual enhancement supplement, Super Power Khan was found to contain sildenafil and hydroxyhomosildenafil.
Recalls & Warnings
July 23, 2016
Seller of GlucoCor Warned for Drug Claims
On June 30, 2016, the FDA issued a warning letter to MC-COR, LLC, following a review of the company's website and Facebook page which found statements made about its GlucoCor capsules to be drug claims.
Recalls & Warnings
August 03, 2018
Vanilla Almond Breeze Almond Milk Recalled
On August 2, 2018, HP Hood LLC issued a recall of half-gallon (1.89 L) cartons of refridgerated Vanilla Almond Breeze almond milk because the product may contain milk, an allergen not listed on the label.
Recalls & Warnings
July 18, 2018
Maker of Vitamin B12 and Multimineral Supplements Warned by FDA
On July 6, 2018, the FDA issued a warning letter to Aegle Nutrition, LLC, following a facility inspection which found some of the company's products, including Tropical Oasis Ionized Trace Minerals, Tropical Oasis Ultra Methyl B12, and Tropical Oasis Premium Vitamin B12 to be ...
Recalls & Warnings
May 29, 2018
FDA Warns Seller of "Aromatase Inhibitor" Supplement
On May 18, 2018, the FDA issued a warning letter to Performance Nutrition Formulators LLC d.b.a. VMI Sports because its product Arimistane contains Androsta-3,5-Diene-7,17-Dione, an ingredient that the FDA considers to be a new drug and not a dietary supplement ingredient.
Recalls & Warnings
May 22, 2018
FDA Warns Companies Selling "Sun Protection" Supplements
On May 18, 2018, the FDA issued warning letters to four companies selling supplements for sun protection because statements made on product labels, websites and in marketing materials were found to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
April 21, 2018
Triangle Pharmanaturals Expands Recall of Kratom Products
On April 17, 2018, Triangle Pharmanaturals, LLC, expanded its original recall of kratom-containing products to include 26 additional kratom products.
Recalls & Warnings
April 21, 2018
NutriZone Expands Recall of Kratom Products
On April 18, 2018 NutriZone LLC expanded its original recall of kratom-containing products to include 29 additional kratom products.
Recalls & Warnings
April 17, 2018
Seller of Osha and Cayenne Supplements & More Warned for Manufacturing Violations
On March 21, 2018, the FDA issued a warning letter to Secret Garden of Health & Healing, LLC, following a facility inspection which found a number of the company's products, including Osha Root and Cayenne, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
March 23, 2019
DG Health NATURALS Baby Cough Syrup Recalled
On March 20, 2019, Kingston Pharma, LLC issued a recall of all lots of DG/health NATURALS baby Cough Syrup + Mucus because it has the potential to be contaminated with Bacillus cereus/Bacillus circulans.
Recalls & Warnings
February 19, 2019
DG Baby Gripe Water Herbal Supplement Recalled
On February 15, 2019, Kingston Pharma, LLC issued a recall of all lots of DG Baby Gripe Water herbal supplement with organic ginger and fennel extracts due to the presence of an undissolved ingredient, citrus flavonoid.
Recalls & Warnings
February 09, 2019
Herbal Skin Remedy Recalled, Can Cause Serious Injury
On February 8, 2019, McDaniel Life-Line LLC issued a recall of all lots of Indian Herb a product marketed to be taken orally or applied topically, as a remedy for abnormal skin growths.
Recalls & Warnings
November 24, 2018
Dangerous Drug In Supplements for Pain and Anxiety
On November 20, 2018, the FDA announced it has issued warning letters to two companies for the illegal marketing of products labeled as dietary supplements that contain the opioid-like drug tianeptine.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
August 16, 2018
Kratom Recalled Due to Salmonella Risk
On August 14, 2018, Zakah Life, LLC issued a recall of Super Green Maeng Da Premium Kratom powder, Powerful Red Vein Bali Premium Kratom powder, Super Green Maeng Da Premium Kratom capsules, and Powerful Red Vein Bali Premium Kratom capsules because laboratory testing revealed the presence of ...
Recalls & Warnings
February 07, 2013
Growth Factor Supplement For "Size, Strength and Stamina" Recalled Due To Potential Contamination
On January 2, 2013, EonNutra, LLC issued a voluntary recall of specific batches of Soto Supplements Growth Factor Complex 200 GFC 200 (2 fl oz.) liquid dietary supplement due to potential bacterial contamination.
Recalls & Warnings
January 30, 2013
Maker of Cold and Flu Supplement Warned For Making Drug Claims
On January 24, 2013, the FDA and FTC jointly issued a warning letter to Flu and Cold Defense LLC, after a review of the company's website and marketing materials found statements made about the cold and flu dietary supplement GermBullet to be drug claims.
Recalls & Warnings
January 28, 2013
Recall: Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 24, 2013, D&S Herbals, LLC, dba Freedom Trading, announced a voluntary recall of one lot of the male sexual enhancement dietary supplement Super Power because it was found to contain undeclared sildenafil.
Recalls & Warnings
January 24, 2013
Food for Health Warned for Manufacturing Violations and Drug Claims on Supplements
On October 5, 2012, the FDA issued a warning letter to Food for Health International, LLC following a facility inspection which found the company's products, including Activz brand Vitamin D, Potassium Iodine, Organic Vitamin C, Whole 9 (a fruit and vegetable meal replacement shake) Control and VMA ...
Recalls & Warnings
February 20, 2013
FDA Warns USPLabs For Adulteration and Drug Claims
On December 4, 2012 the FDA issued a warning letter to USPLabs, LLC following facility inspections which found the company's products, including dietary supplements Jacked3d, OxyElite Pro, Prime, and Super Cissus, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
February 15, 2013
FDA Seizes Weight Loss Supplements Containing Undeclared Drug
On Feb. 14, 2013, U.S. Marshals acting on behalf of the FDA seized weight loss supplements from Globe All Wellness, LLC which were found to contain the undeclared drug sibutramine hydrochloride.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
February 21, 2013
Pet Supplement Company Issues Recall Due To Potential Bacterial Contamination
On February 20, 2013, animal supplement company Nutri-Vet, LLC issued a recall of Nutri-Vet and NutriPet Chicken Jerky Products because they may be contaminated with Salmonella.
Recalls & Warnings
August 27, 2013
FTC Refunds Consumers of Children's Vitamins as Part of Deceptive Advertising Settlement
On August 8, 2013, the Federal Trade Commission (FTC) announced it has mailed 10,144 refund checks to consumers who purchased Disney or Marvel Hero -themed children's vitamins, including Disney Princesses, Winnie the Pooh, Finding Nemo, and Spider-Man varieties, between May 1, 2008 and September ...
Recalls & Warnings
August 27, 2013
Immune Supplement Recalled Due To Undeclared Milk
On August 23, 2013, Reaction Nutrition, LLC issued a voluntary recall of immune support supplement LIVE CLINICAL 90 CAPS because it contains undeclared milk.
Recalls & Warnings
November 20, 2013
Another Sexual Enhancement Supplement Recalled Due To Undeclared Drugs
On November 18, 2013, Fossil Fuel Products, LLC issued a voluntary recall of sexual enhancement supplement RezzRX because it was found to contain the undeclared drugs hydroxylthiohomosildenafil and aminotadalafil.
Recalls & Warnings
November 20, 2013
OxyElite Pro Recall Expanded
On November 19, 2013, USPlabs LLC expanded its recall of OxyElite Pro products to include Raspberry Lemonade OxyELITE Pro Super Thermo Powder (4.6 oz., UPC #094922447494).
Recalls & Warnings
July 23, 2013
Weight Loss Supplements Containing Undeclared Drugs Recalled
On July 19, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss supplements Esbelin siloutte te and Esbelin siloutte Herbal Blend with L-Carnitine because they were found to contain undeclared sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
October 27, 2013
Ingredient In Workout Supplements May Be Unsafe, FDA Warns
On October 11, 2013, the FDA directed USP Labs, LLC to cease distribution of workout supplement OxyElite Pro and and muscle-building supplement VERSA-1, which are labeled as containing the ingredient aegeline, or N-[2-hydroxy-2(4-methoxyphenyl) ethyl]-3-phenyl-2-propenamide.
Recalls & Warnings
October 01, 2013
Three Sexual Enhancement Supplements Containing Undeclared Drug Recalled
On September 24, 2013, Haute Health, LLC issued a voluntary recall of all lots of sexual enhancement supplements Virilis Pro, PHUK and Prolifta, because they were found to contain sildenafil.
Recalls & Warnings
September 15, 2013
Creatine Powder Containing DMAA Recalled
On September 12, 2013, Ge Pharma, LLC issued a recall of grape and fruit punch flavors of Creafuse Powder because they contain 1,3 dimethylamylamine (DMAA).
Recalls & Warnings
May 10, 2013
Seller Of Sexual Enhancement and Hormonal Balance Supplements Warned For Manufacturing Violations
On April 26, 2013, the FDA issued a warning letter to Pristine Bay, L.L.C.
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
May 01, 2014
Weight Loss Supplement Recalled
On April 29, 2014, dietary supplement distributor Bacai Inc. issued a voluntary recall of one lot of weight loss supplement LiteFit USA because it was found to contain sibutramine.
Recalls & Warnings
March 27, 2014
Bee Pollen and Weight Loss Supplements Recalled
On March 26, 2014, Pure Edge Nutrition, LLC issued a voluntary recall of Bella Vi Insane Bee Pollen Capsules, Bella Vi BTrim Ultimate Boost, Bella Vi BTrim Max, Bella Vi Extreme Accelerator, Bella Vi Insane Amp'd, and two lots of Bella Vi Amp'd Up because they were found to contain sibutramine, ...
Recalls & Warnings
February 17, 2014
Weight Loss Supplement Recall Expanded to Include Additional Products
On February 4, 2014, MyNicKnaxs, LLC, which first issued a recall of Reduce Weight Fruta Planta, issued a recall of nine additional weight loss supplements: Magic Slim, Fruta Bio, SlimEasy, Super Fat Burning Bomb, Slim Xtreme, Meizi Evolution, Meizitang Strong Version Botanical Slimming, ...
Recalls & Warnings
February 14, 2014
Weight Loss Supplement Containing Drug Recalled
On February 4, 2014, MyNicKnaxs, LLC. issued a recall of weight loss supplement Reduce Weight Fruta Planta because it was found to contain phenolphthalein.
Recalls & Warnings
February 13, 2014
Seller of Iron, Prostate, Valerian and Other Supplements Warned For Multiple Violations and Drug Claims
On January 31, 2014, the FDA issued a warning letter to NatureAll-STF Holding, LLC, following a facility inspection which found the company's products, including Colostrum, Valerian-Plus, Wormwood, Black Walnut-Plus, Lung-Plus, Yucca-Plus (Arthro Plus), Iron Plus, MSM Plus, White Willow Plus, ...
Recalls & Warnings
October 30, 2014
Supplement Maker Warned for Manufacturing Violations
On October 21, 2014, the FDA issued a warning letter to DNG Trading & Milling, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
October 24, 2014
Shark Cartilage Supplement Recalled
On October 17, 2014, AMS Health Sciences, LLC issued a voluntary recall of one lot of Saba Shark Cartilage Complex because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
July 25, 2015
Weight Supplement Found to Contain Three Drugs
On July 23, 2015 Life & More, L.L.C. issued a voluntary recall of 783 bottles from one lot of Akttive High Performance Fat Burner Gold weight loss capsules because they were found to contain the undeclared drugs sibutramine, desmethylsibutramine, and phenolphthalein.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
September 13, 2014
Marketer Banned From Selling Weight Loss Products
In order to settle FTC charges of deceptive weight loss claims, John Matthew Dwyer III, the co-founder of HealthyLife Sciences, LLC, has agreed to no longer manufacture or market weight loss supplements.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
July 02, 2014
Protein Drink Mix Recalled Due to Salmonella Risk
On July 1, 2014, Oriya Organics, LLC voluntarily recalled Oriya Organics Superfood Protein Medley (21.2 oz) because it contains Organic Sprouted Chia Seed Powder, which has the potential to be contaminated with Salmonella.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 12, 2014
Marketers of HCG Settle FTC Charges of Deceptive Weight Loss Claims
Marketers of HCG Platinum drops have agreed to pay $1 million to settle Federal Trade Commission (FTC) charges that claims the drops could cause rapid and substantial weight loss were deceptive and not supported by scientific evidence.
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
November 21, 2014
Weight Loss Supplements Recalled
On November 19, 2014, REFA Enterprises, LLC issued a voluntary recall of one lot each of Forever Beautiful Bee Pollen and Forever Beautiful Infinity because they were found to contain sibutramine or a combination of sibutramine and phenolphthalein.
Recalls & Warnings
November 11, 2014
Seller of Sexual, Muscle Enhancement Supplements Warned for Manufacturing Violations and Drug Claims
On August 26, 2014, the FDA issued a warning letter to GE Pharma LLC following a facility inspection which found the company's products, including Fire Burn, Fire Storm, Creatine, Amino Fire, Nitric Fire, Jet Fire, Oxy Fire, HGH, Cissus, Hydro shield, Performa-Test, Raspberry Ketones, Fire Drol, ...
Recalls & Warnings
April 11, 2015
Acai Berry Marketers Who Tricked Consumers with Fake News Sites Ordered to Pay $16 Million
On April 6, 2015, the Federal Trade Commission (FTC) announced that a recent U.S. district court ruling will require marketing affiliates for LeanSpa's acai berry and "colon cleanse" weight loss products to pay $16 million in consumer redress.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
February 17, 2015
Seller of Muscle Supplements Containing Synthetic Steroids Warned
On February 9, 2015, the FDA issued a warning letter to A2Z Industries, LLC, following a facility inspection which found the company's muscle enhancing supplements, including HaloV and EPI2A3A to be labeled as containing synthetic steroids.
Recalls & Warnings
February 06, 2015
Seller of Aloe Supplement Warned for Drug Claims
On January 21, 2015, the FDA issued a warning letter to Pristine Nutraceuticals, LLC, following a review of the company's website, http://www.digestaqure.com, which found statements made about DigestaCure to be drug claims.
Recalls & Warnings
January 27, 2015
Green Coffee Bean Supplement Marketer Settles FTC Charges of Deceptive Claims
On January 26, 2015, the FTC announced that Pure Health LLC, Genesis Today, and owner of both companies, Lindsey Duncan, will pay $9 million to settle charges of deceptive weight loss claims made about the companies' green coffee bean extract products.
Recalls & Warnings
January 11, 2012
Herbal Extract Company Warned by FDA of Making Drug Claims
On January 3, 2012, the U.S. FDA sent a Warning Letter to Herbal Extract Plus, LLC regarding legal violations for claiming its herbal products can cure or prevent diseases.
Recalls & Warnings
March 08, 2012
Rx Drugs Found in Weight Loss and Enhancement Supplements
On February 6, 2012, FDA issued a letter to Globe All Wellness, LLC warning that two of their products contain undeclared prescription drugs.
Recalls & Warnings
November 06, 2012
Recall of "Bee Pollen" Supplement Found to Contain Drug
On October 24, 2012, Zi Xiu Tang Success, LLC issued a voluntary recall of Classic Zi Xiu Tang Bee Pollen Capsules and Ultimate Formula Capsules because the supplements contain the undeclared drug sibutramine.
Recalls & Warnings
September 26, 2012
Maker of Eye Health Supplements Warned for Drug Claims
On September 18, 2012, the FDA issued a warning letter to EyeScience Labs, L.L.C. because statements made about the company's Macular Health Formula, Dry Eye Formula and Diabetic Vision Formula supplements were found to constitute drug claims.
Recalls & Warnings
September 12, 2006
U.S. Marshalls Seize Supplements Promoted as Drugs
On September 6, 2005, the U.S. Food and Drug Administration (FDA) announced that, at its request, U.S. Marshals seized quantities of Ellagimax capsules, Coral Max capsules, Coral Max without Iron capsules, and Advanced Arthritis Support capsules distributed by Advantage Nutraceuticals L.L.C.
Recalls & Warnings
April 07, 2008
Federal Agents Seize Nearly $1.3 Million of Illegal Dietary Supplements
On April 4, 2008 the U.S. FDA announced that, at its request, U.S. Marshals had seized more than $1,301,712 of dietary supplements from LG Sciences, LLC, of Brighton, Mich.
Recalls & Warnings
May 13, 2009
Body-Building Supplements Confiscated by FDA for Adulteration
On May 11, 2009, the U.S. FDA announced that U.S. Marshalls seized more than 23,300 bottles of three dietary supplement products distributed by LG Sciences LLC, of Brighton, Mich.
Recalls & Warnings
July 30, 2008
Recall of Viapro Capsules Due to Potentially Harmful Ingredient
The U.S. FDA has posted a release noting that EG Labs, LLC announced a nationwide voluntary recall on July 23, 2008 of all lots of its supplement product sold under the brand name, Viapro, in 375mg capsules.
Recalls & Warnings
March 04, 2011
Recall of "Raw" Vitamin C Supplement Containing Soy
The FDA posted a notice dated March 2, 2011 from Garden of Life, LLC regarding that company's voluntary recall of its Raw Vitamin C supplement because it may contain undeclared soy proteins.
Recalls & Warnings
January 04, 2011
Weight Loss Supplement Containing a Drug Can Cause Serious Adverse Events, Warns FDA
On December 31, 2010 the U.S. FDA warned consumers not to use Fruta Planta weight loss products because they contain sibutramine, a drug withdrawn from the market in December 2010 for safety reasons.
Recalls & Warnings
December 14, 2010
FDA Warns Consumers to Avoid Man Up Now Capsules
On December 14, 2010, the U.S. FDA warned consumers not to use Man Up Now capsules, marketed as a dietary supplement for sexual enhancement, because they contain a variation of an active drug ingredient found in Viagra that can dangerously lower blood pressure.
Recalls & Warnings
November 06, 2009
FDA Warns that "Stiff Nights" Enhancement Supplement Contains Undeclared Drug
On November 5, 2009 the U.S. Food and Drug Administration (FDA) warned consumers that Stiff Nights, a product marketed as a dietary supplement for sexual enhancement, contains an ingredient that can dangerously lower blood pressure and is illegal.
Recalls & Warnings
May 05, 2003
FDA Reports False Claims by Maker of HGH Supplement
On April 30, 2003, the Food and Drug Administration (FDA) announced that Nature's Youth, LLC, of Centerville, Mass., has completed its voluntarily destruction of approximately 5700 boxes (each containing a 30-day supply) of its misbranded product, "Nature's Youth hGH.
Recalls & Warnings
March 13, 2006
FDA Warns About Steroid Products Sold as Dietary Supplements
On March 9, 2006, the U.S.
Recalls & Warnings
March 16, 2018
FTC Sends Refund Checks to Consumers of Unproven Weight-Loss Products
On March 15, 2018, the FTC announced it is mailing 18,301 checks totaling more than $437,000 to consumers who purchased weight-loss products from Colby Fox, Christopher Reinhold, and their companies, Tachht, Inc. and Teqqi, LLC.
Recalls & Warnings
March 06, 2018
Protein Bars Recalled Due to Undeclared Allergens
On March 5, 2018, IDLife, LLC, issued a recall of their Protein Bars, Snack Bars, and Kids Bars because they may contain undeclared almonds, peanuts, and/or coconut oil.
Recalls & Warnings
June 21, 2016
"Doctor Trusted" Seal and Certification Program for Dietary Supplements Misleading and Meaningless, Says FTC
SmartClick Media LLC, also doing business as Doctor Trusted, has agreed to settle Federal Trade Commission (FTC) charges that its "Doctor Trusted" seal and certificates, which appeared on some 800 websites promoting health products and dietary supplements, ...
Recalls & Warnings
June 18, 2016
Muscle Milk Protein Drinks Recalled
On June 17, 2016 HP Hood LLC issued a recall of the following Muscle Milk protein drinks because they have the potential for premature product spoilage:
Recalls & Warnings
June 09, 2016
Nature Made Multis and B Complex Recalled Due to Salmonella, Staph Risk
On June 8, 2016 Pharmavite LLC issued a recall of the following Nature Made® multivitamin and B complex supplements because they have the potential to be contaminated with Salmonella or Staphylococcus aureus:
Recalls & Warnings
May 15, 2016
Seller of Vision Supplements Warned for Drug Claims
On April 28, 2016, the FDA issued a warning letter to Macular Health, LLC.
Recalls & Warnings
May 02, 2016
Weight Loss Supplements Containing Hidden Drugs Recalled
On April 29, 2016 Making It A Lifestyle, L.L.C. issued a recall of all lots of weight loss supplements 3rd Degree, Black Gold X Advanced and Black Label X because they were found to contain undeclared sibutramine and sildenafil.
Recalls & Warnings
April 05, 2016
Muscle Enhancement Supplement Recalled
On April 5, 2016, Invisiblu International LLC issued a recall of one lot of the muscle enhancement supplement Continuum Labs LGD-Xtreme because it contains LGD-4033 Ligandrol. The risks of this ingredient are not known.
Recalls & Warnings
February 06, 2016
Marketers of Weight Supplements AF Plus and Final Trim Violated Consumer Protection Laws, Says FTC
On January 19, 2016, the Federal Trade Commission (FTC) and State of Maine's Office of the Attorney General charged marketers of weight loss supplements AF Plus and Final Trim with violating consumer protection laws.
Recalls & Warnings
February 05, 2016
Garcinia, Green Coffee Marketers Pay $43 Million to Settle Charges of False Weight Loss Claims
Sale Slash, LLC, has agreed to pay more than $43 million to settle FTC charges it used unsubstantiated claims and fake celebrity endorsements to promote its Premium Green Coffee, Pure Garcinia Cambogia, Premium White Kidney Bean Extract, Pure Forskolin Extract, and Pure Caralluma Fimbriata Extract ...
Recalls & Warnings
March 04, 2016
Federal Court Shuts Down Seller of Supplement Promoted for Herpes
On February 26, 2016, a federal court ordered dietary supplement company Viruxo LLC to stop selling its product Virux, which was promoted to treat herpes.
Recalls & Warnings
March 02, 2016
Seller of Hoodia and Other Supplements Admits Fraud
On March 1, 2016, The United States Department of Justice announced that David Romeo, principal of several New Jersey- based dietary supplement companies, including Global Nutrients, Stella Labs and Nutraceuticals International, LLC, has pled guilty to conspiracy to distribute three kilograms or ...
Recalls & Warnings
February 16, 2016
Work Out and Weight Supplements Contain Synthetic Amphetamine-Like Compound
On February 3, 2016, the FDA issued a warning letter to ATS Labs, LLC because labels for the company's work out and weight loss supplements CFI, Weapon-X Pre-Workout Extreme, and Lady Lean list a synthetic, amphetamine-like compound, 4-amino-2-methylpentane citrate (also called ...
Recalls & Warnings
February 16, 2016
Garden Of Life Recall Expanded
On February 13, 2016, Garden of Life LLC expanded its January 2016 recall of certain Raw Meal Organic Shake & Meal Chocolate, Original, Vanilla and Vanilla Chai products to include additional lots of these products, because they have the potential to be contaminated with ...
Recalls & Warnings
January 20, 2016
Seller of Aloe, Moringa Supplements Warned for Manufacturing Violations, Drug Claims
On January 13, 2016, the FDA issued a warning letter to Alkebulan International Services, LLC, following a facility inspection which found the company's products,Aloe Ferox and Moringa Oleifera Capsule to be adulterated because they prepared, packed, or held under conditions that ...
Recalls & Warnings
January 12, 2016
Over $400,000 Worth of Kratom Supplements Seized by U.S. Marshals
On January 6, 2016, U.S. Marshals seized nearly 90,000 bottles of dietary supplements labeled as containing kratom (brand name RelaKzpro) valued at over $400,000, from Dordoniz Natural Products LLC in South Beloit, Illinois. The action was taken at the request of the FDA.
Recalls & Warnings
November 10, 2015
"Natureal" Weight Supplement Recalled
On November 9, 2015 Inaffit, LLC issued a voluntary recall of all lots of weight loss supplement Natureal because it was found to contain undeclared sibutramine.
Recalls & Warnings
August 23, 2015
Maker of Herbal Supplements Sold on eBay, Amazon and Other Websites Shut Down
On August 17, 2015, a federal court ordered a permanent injunction against dietary supplement company Iowa Select Herbs LLC for unlawfully manufacturing and distributing unapproved new drugs and misbranded drugs and adulterated and misbranded dietary supplements.
Recalls & Warnings
September 30, 2015
Weight and Energy Supplement Contains Synthetic "Amphetamine"
On September 21, 2015, the FDA issued a warning letter to TruVision Health LLC. because the company's supplement tru Weight & Energy lists a synthetic, amphetamine-like compound, AMP (also called 1,3-dimethylbutylamine or DMBA) on product labels.
Recalls & Warnings
December 23, 2015
"Bee Extremely Amazed" Recalls Weight Supplements
On December 22, 2015, Bee Extremely Amazed LLC issued a voluntary recall of all lots of the following weight loss supplements, which were found to contain sibutramine and phenolphthalein:
Recalls & Warnings
April 01, 2017
Maker of Prelief, Urinozinc Prostate Health Formula Warned for Manufacturing Violations
On March 16, 2017 the FDA issued a warning letter to DSE Healthcare Solutions, LLC, following a facility inspection which found the company's products, including Prelief and Urinozinc Prostate Health Formula to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
October 13, 2016
Nutrisystem Bars Recalled Due to Listeria Risk
On October 11, 2016, Nutrisystem Everyday, LLC issued a recall of one lot of its Nutricrush Chocolate Chip Cookie Dough bar because it has the potential to be contaminated with Listeria monocytogenes.
Recalls & Warnings
February 16, 2017
U.S. Department of Justice Files Permanent Injunction Against Supplement Manufacturer
On February 16, 2017, the United States Department of Justice filed a complaint against Pick and Pay, Inc.
Recalls & Warnings
January 07, 2017
Seller of Protein and Workout Supplements Warned for Manufacturing Violations, Label Errors
On December 22, 2016, the FDA issued a warning letter to Rock Solid Nutrition, LLC, following a facility inspection which found the company's products, including Whey Isolate (Cinnabun flavor), Pre-Pump (Massive Mango flavor) and Strength Test to be adulterated because ...
Recalls & Warnings
August 02, 2016
Seller of Whey Protein Warned for Manufacturing Violations, Drug Claims
On July 22, 2016, the FDA issued a warning letter to New Horizon Nutraceuticals, LLC, following a facility inspection which found the company's product, One World Whey Protein Power Food to be adulterated because it was prepared, packed, or held under conditions that violate Current ...
Recalls & Warnings
August 09, 2016
Liquid Multis, Vitamin D & More Recalled Due to Potential Bacterial Contamination
On August 8, 2016, PharmaTech, LLC issued a recall of twenty liquid vitamin supplements, sold under various brand names, because they have the potential to be contaminated with Burkholderia cepacia.
Recalls & Warnings
July 18, 2017
Seller of "Super Strength Prostate Formula," Acai Berry Supplements & More Warned for Drug Claims
On June 30, 2017, the FDA issued a warning letter to Nature's Health Company, LLC, following a facility inspection and review of the company's website, www.natureshealthcompany.
Recalls & Warnings
June 07, 2017
FDA Warns Seller of Probiotic and Omega-3 Supplements for Manufacturing Violations
On May 22, 2017, the FDA issued a warning letter to BioTE Medical, LLC following a facility inspection which found its products, including BioTE DIM, BiotTE Probiotic, BioTE Iodine Plus, and BioTE Omega 3 to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
May 30, 2017
Beef Bone Broths Recalled
On May 26, 2017, Cauldron Soups, LLC (DBA Cauldron Broths) issued a recall of approximately 5,163 pounds of beef broth products because they were produced without the benefit of federal inspection.
Recalls & Warnings
May 26, 2017
"Allergy" Supplement Containing Ephedra Recalled
On February 7, 2017, MusclMasster, LLC (DBA The Green Herb) of Wheat Ridge, CO issued a recall of all bottles of Al-Er-G Capsules , a product promoted to help allergies, because they contain ephedra.
Recalls & Warnings
May 20, 2017
Muscle Enhancement Supplement Containing Steroid-Like Substances Recalled
On May 19, 2017, Dynamic Technical Formulations LLC issued a recall of all lots its muscle enhancement supplement Tri-Ton because it was found to contain anabolic steroid-like substances (andarine and ostarine) which are not permitted in dietary supplements.
Recalls & Warnings
October 07, 2017
Weight Loss Supplement Recalled
On October 5, 2017, Kiriko, LLC recalled recalled all lots of A1 Slim 30 capsules after FDA analysis found the products to contain sibutramine, phenolphthalein and N-Desmethyl sibutramine.
Recalls & Warnings
February 10, 2018
Warning to D-Limonene, Vitamin C Seller
On January 31, 2018 the FDA issued a warning letter to Long Life Unlimited, LLC following a review of the company's website which found promotional statements and testimonials made about products including Balance 600, D-Limonene, Rapha Remedy, Rapha Remedy w/ p73 Wild Oregano, Vitamin C, ...
Recalls & Warnings
February 02, 2018
Seller of Vitamin D, Omega-3's, Whey Protein & More Warned for Drug Claims
On January 24, 2018 the FDA issued a warning letter to Young Health Products, LLC following a review of the company's website which found promotional statements and testimonials made about the certain products, including Lugol's Solution 2%, Liquid Vitamin D3, Probimune, Flax Seed & Omega ...
Recalls & Warnings
January 21, 2018
Joint Supplement Recalled for Salmonella Risk
On January 21, 2018, Arthri-D, LLC announced a recall of its joint pain supplement, Arthri-D (120 count), due to possible Salmonella contamination.
Recalls & Warnings
January 19, 2018
Flawless Beauty Shut Down, Company's Kits and Products Recalled
On January 19, 2018, Flawless Beauty, LLC issued a recall for all lots of nineteen products sold individually or together as part of its "whitening kits."
Recalls & Warnings
January 16, 2018
Marketers of CellAssure and Cognify Settle FTC Charges of Deceptive Cancer Claims
On January 11, 2018, the FTC announced that CellMark Biopharma, LLC and its CEO, Derek E.
Recalls & Warnings
January 13, 2018
Seller of Depression, Prostate Supplements & More Warned for Manufacturing Violations, Drug Claims
On August 21, 2017, the FDA issued a warning letter to Irmo-Vita, LLC, following a facility inspection which found that several of the company's products, including Hysta-Min, Man-Affirm, Phung-EX, and Bacto- EX to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
December 26, 2017
Seller of BrainAlert Warned for Manufacturing Violations, Drug Claims
On December 14, 2017, the FDA issued a warning letter to BrainAlert, LLC.
Recalls & Warnings
December 01, 2017
Health Research Labs Agrees to Settle FTC Charges of False Claims, Deceptive Marketing of BioTherapex and NeuroPlus
On November 30, 2017, the FTC announced Health Research Laboratories LLC and owner, Kramer Duhon, agreed to settle charges that they made false claims about the company's products, BioTherapex and NeuroPlus.
Recalls & Warnings
June 23, 2017
FDA Warns Seller of Menopause, Sexual Enhancement, Prostate Supplements and More For Manufacturing Violations
On May 26, 2017 the FDA issued a warning letter to Star Health & Beauty LLC, following a facility inspection which found the company's products, including Nu Essentials Royal Jelly Capsules, NuMan Male Enhancement Capsules, Star's Male Potency Tonic, NuGen HP, She Max HP, and V Max ...
Recalls & Warnings
February 25, 2017
Radio "Infomercials" for Cognitive and Joint Health Supplements Deceived Consumers, Says FTC
On February 22, 2017, the Federal Trade Commission and the Maine Office of the Attorney General announced a complaint the marketers of CogniPrin and FlexiPrin, charging that they made misleading claims about the supplements in radio infomercials deceptively formatted as talk shows.
Recalls & Warnings
August 18, 2017
FDA Warns Consumers Not to Use Certain Liquid Multis, Vitamin D, and Other Supplements Due to Potential Bacterial Contamination
On August 11, 2017, the FDA advised the public not to use any liquid drug or dietary supplement products manufactured by PharmaTech LLC of Davie, Florida, and labeled by Rugby Laboratories, Major Pharmaceuticals, and Leader Brands, because they have the potential to be contaminated with ...
Recalls & Warnings
December 16, 2015
Maker of Red Yeast Rice, St. John's Wort, Valerian and More Warned for Manufacturing Violations, Drug Claims
On December 2, 2015, the FDA issued a warning letter to Nature's Health, LLC, following a facility inspection which found the company's product, including Ginkgo & Rhodiola, Blood Sugar Balance IV, Cinnamon Extract, Choles-Balance Red Yeast Extract, Lecithin, Milk Thistle Seed Extract, ...
Recalls & Warnings
August 07, 2015
Male Enhancement and Weight Loss Supplement Containing Drugs Recalled
On August 6, 2015 Blue Square Market Inc. issued a recall of the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
October 28, 2015
GNC Accused of Selling Supplements with Unlawful Ingredients
On October 22, 2015, Oregon Attorney General Ellen Rosenblum filed a lawsuit against supplement retailer GNC, alleging that the company sold products that were adulterated with BMPEA and picamilon, which are not lawful dietary supplement ingredients.
Recalls & Warnings
October 08, 2015
FDA Warns Seller of Omega-3 "Concussion" Supplement for Drug Claims
On October 1, 2015, the FDA issued a warning letter to MPH Nutrition, LLC, following a review of the company's website which found statements made about Re:Mind Recover, a supplement promoted for recovery from concussions, to be drug claims which are not permitted for supplements.
Recalls & Warnings
February 01, 2016
Garden of Life Shakes and Meals Recalled Due to Salmonella Risk
On January 29, 2016, Garden of Life LLC issued a recall of certain Raw Meal Organic Shake & Meal Chocolate, Original, Vanilla and Vanilla Chai products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
February 27, 2018
Dog Treats, Food Recalled Due to Salmonella, Listeria Risk
The following dog treats and dog food are being recalled because they have the potential to be contaminated with Listeria monocytogenes or Salmonella, which can cause illness in pets who consume these products, as well as in pet owners who handle the contaminated products or touch ...
Recalls & Warnings
April 03, 2018
FDA Issues Mandatory Recall of Salmonella-Contaminated Kratom Products
On April 2, 2018, the FDA issued a mandatory recall of all food products containing powdered kratom manufactured, processed, packed, or held by Triangle Pharmanaturals LLC because several of company's products were found to be contaminated with Salmonella.
Recalls & Warnings
April 02, 2018
NutriZone Kratom Supplements Recalled Due to Salmonella Risk
On March 10, 2018, NutriZone, LLC issued a recall of certain kratom-containing powder products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
March 31, 2018
Nutrition Bars Sold at Whole Foods and Online Recalled
On March 26, 2018, eBars LLC, issued a recall of their Protein Bars, Snack Bars, and Kids Bars because they may contain undeclared peanuts and/or almonds.
Recalls & Warnings
January 18, 2005
Sellers of “Fat Trapper Plus” and “Exercise in a Bottle” Banned from Advertising Weight-Loss Products
On January 18, 2005, the Federal Trade Commission (FTC)announced that Enforma Natural Products, Inc.
Recalls & Warnings
November 03, 2009
Recall of 65 Dietary Supplements That May Contain Steroids
On November 3, 2009, as part of its ongoing cooperation with the Food and Drug Administration ("FDA"), Bodybuilding.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
May 30, 2015
Seller of Omega-3, Probiotics & SuperFoods Warned for Manufacturing Violations, Drug Claims
On May 4, 2015, the FDA issued a warning letter to Dr. Dennis Black, LLC.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
September 26, 2014
Seller Warned for Promoting Essential Oils to Treat Flu, MRSA, Measles and More
On September 22, 2014, the FDA issued a warning to doTERRA International, LLC.
Recalls & Warnings
January 09, 2014
Four Companies Settle FTC Charges of Deceptive Weight Loss Claims
On January 7, 2014, the Federal Trade Commission (FTC) announced that marketers for four weight loss products have agreed to settlements over charges of deceptive weight loss claims.
Recalls & Warnings
November 11, 2013
Workout Supplement OxyElite Pro Recalled After Being Linked to Serious Liver Illness
On November 10, 2013, the FDA announced that USPLabs LLC is recalling workout supplements OxyElite Pro Super Thermo capsules, OxyElite Pro Ultra-Intense Thermo capsules and OxyElite Pro Super Thermo Powder because they have been linked to a number of cases of acute non-viral hepatitis, one of which ...
Recalls & Warnings
December 06, 2013
Weight Loss Supplement Claims Challenged
On November 27, 2013, the National Advertising Division (NAD) recommended HealthyLife Sciences, LLC, modify or discontinue the use of certain claims made about the company's weight loss supplement Healthe Trim.
Recalls & Warnings
August 20, 2013
Weight Loss Supplement Formulas For Men and Women Recalled Due To Undeclared Drugs
On August 16, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss dietary supplements Esbelder man, Esbelder fem and Esbelder siloutte because they were found to contain the undeclared drugs sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
April 26, 2013
Seller of Immune, Cholesterol, Liver and Blood Sugar Products Warned For Drug Claims
On March 20, 2013, the FDA issued a warning letter to Birkdale Medicinals LLC, following a review of the company's website which found statements made about Birkdale products, including Immune Response 247, Cholestat, Silymarin 81 and LevelStat, to be drug claims.
Recalls & Warnings
April 04, 2013
Three More Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On April 3, 2013, the FDA warned consumers that the sexual enhancement supplements AFFIRM XL, Love Rider and Ninja Mojo were found to contain undeclared erectile dysfunction drugs. Consumers are urged to stop using these supplements immediately.
Recalls & Warnings
February 21, 2013
FDA and FTC Warn: Supplements Cannot Prevent, Treat Or Cure Cold And Flu
On February 11, 2013, the FDA and the Federal Trade Commission (FTC) jointly issued warning letters to three dietary supplement companies regarding their potential illegal marketing of products to prevent, treat or cure flu virus.
Recalls & Warnings
November 29, 2012
Sensa Settles Second False Advertising Lawsuit
On November 27, 2012, Sensa Products LLC, maker of the Sensa Weight Loss System, announced it agreed to settle a false advertising lawsuit filed by the Nutritional Supplemental Task Force in California, without an admission of guilt.





