Reviews and Information for CBD
CBD & Hemp Extract Supplements
Tests, comparisons, and reviews of CBD oils, softgels, gummies and topicals by ConsumerLab. See CL's Top Picks for best quality, lowest cost CBD. Learn what to look for on labels when choosing CBD and information on what CBD does and doesn't do, dosage, and side effects.
CBD & Hemp Extract Supplements ReviewSearch term may appear only in full report available to members. Join now for full access.
CL Answer
Can CBD cause a drop in blood pressure?
Find out if CBD (cannabidiol) can lower blood pressure or affect heart rate, plus information about other CBD side effects, drug interactions and safety concerns. ConsumerLab.com's answer explains.
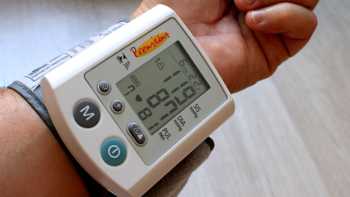
CL Answer
Can hemp oil or CBD (cannabidiol) supplements cause me to fail a marijuana drug test?
Find out if you can fail a drug test dues to hemp oil or CBD (cannabidiol) supplements. Plus info on how much THC is in hemp oil and CBD products. ConsumerLab.com's answer explains.
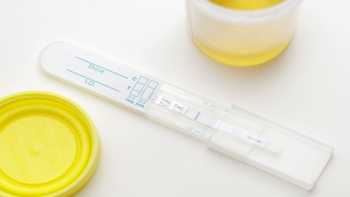
CL Answer
Do any supplements help with COVID-19? Do supplements like vitamin D, zinc, vitamin C, or herbals work?
Find out if natural remedies & supplements for coronavirus such as zinc, vitamin C, garlic, or elderberry help to prevent or treat COVID-19.
News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
Recalls & Warnings
November 30, 2023
Discover Health, LLC Warned for Promoting CBD Products for Cancer, Epilepsy, & More
On November 16, 2023, the FDA issued a Warning Letter to Discover Health, LLC d/b/a Discover CBD and Strain Snobs following a review of the company’s websites that found statements about the company’s Active CBD Oil – Full Spectrum Distillate Cartridge, Active CBD Oil – ...
News Release
August 23, 2023
ConsumerLab Tests Find More CBD, Less THC in CBD Oils, Gummies, and Topicals
White Plains, New York, August 23, 2023 — Recent ConsumerLab tests of popular CBD (cannabidiol) products on the market revealed that, overall, products contain more CBD, and less THC (tetrahydrocannabinol, a psychoactive compound) than in previous years.
Recalls & Warnings
November 23, 2022
CBD Not Permitted in Gummies, Candies, Cookies, Candy, or Pet Treats, Says FDA
On November 16, 2022, the FDA issued warning letters to five companies for selling products such as gummies, tea, cookies, lollipops, fruit snacks, hard candies, and pet treats containing cannabidiol (CBD) and/or delta-8 tetrahydrocannabinol (delta-8 THC) as conventional food products.
Clinical Update
6/19/2018
Cannabidiol (CBD) Side Effects
A CL Member recently reported developing "dry mouth" after using CBD oil. Dry mouth is among the potential side effects of CBD. Learn more about this and other CBD side effects in the Concerns and Cautions section of the CBD & Hemp Supplements Review. (Also see our Top Picks among CBD products.)
Clinical Update
8/22/2019
A Better CBD?
A formulation of CBD was shown to increase CBD levels in the blood significantly more than the same amount of CBD in oil. But is this any better than simply taking regular CBD with a meal? Find out in the How to Take section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Recalls & Warnings
November 26, 2019
FDA Warns Companies Selling CBD Products as Dietary Supplements
On November 25, 2019, the FDA issued warning letters to 15 companies for selling products containing CBD (cannabidiol) labeled and marketed as dietary supplements, and/or for making drug claims about these products.
Recalls & Warnings
April 28, 2020
Natures CBD Oil Distribution Warned for Joint Pain Claims
On April 20, 2020, the FDA issued a warning letter to Homero Corp DBA Natures CBD Oil Distribution, following an inspection of the company's website which found the company's products, including Natures Pure CBD Oil, CBD Froggies 25mg, CBD Froggies 50mg, CBD Edibles 200mg Frogs, CBD ...
CL Answer
Which supplements and lifestyle changes improve sleep, and which cause insomnia?
Find out which vitamins may help you sleep and learn if any vitamins or supplements can cause insomnia.

Clinical Update
11/27/2018
Side Effects of CBD
High-dose CBD (cannabidiol) often causes side effects. In fact, adverse events were reported in 81% of participants in a recently reported CBD study. Get the details in the Concerns and Cautions section of the CBD Oil and Hemp Extract Review. (Also see our Top Pick for CBD.)
Clinical Update
8/11/2018
CBD for Dogs
Can CBD help dogs with osteoarthritis (worn joints)? Learn what a recent study found (as well as the dose used and a potential side effect) in the CBD for Pets section of the CBD & Hemp Supplements Review. (Also see our Top Picks for CBD for pets – as well as for people).
Clinical Update
2/26/2019
Does CBD Cream Help?
Does CBD cream decrease muscle soreness following exercise? Does CBD gel help with knee pain from osteoarthritis? Learn what recent studies have found in the What It Does section of the CBD and Hemp Extract Review. Also see our Top Picks for CBD oils and topical products.
Clinical Update
8/11/2020
CBD Reduces Cannabis Use
CBD can reduce cannabis use, but only when taken at the right dose, according to a recent study. Find out what dose of CBD helped most in the What It Does section of the CBD & Hemp Extract Supplements Review. Also see our Top Picks for CBD.
Clinical Update
1/03/2020
CBD Cream for Pain?
People suffering peripheral neuropathy in their legs and feet applied CBD cream or placebo for one month. Did it help? Find out in the Topical CBD section of our CBD Review. Also see our Top Picks among creams, oils, and other CBD products.
Clinical Update
5/23/2020
CBD Label Inaccuracies
Tests of CBD products purchased at local stores showed that many provided much less CBD than claimed. Get the details in the Products on the Market section of the CBD and Hemp Extracts Review. Also see our Top Picks for CBD products.
Clinical Update
6/09/2018
Danger With Fake CBD Oil
A synthetic form of cannabidiol appears to be responsible for dozens of reports of adverse effects occurring shortly after taking certain CBD oil products. For details, see the Quality Concerns section of our Review. Also see our Top Picks among CBD and hemp extract products.
Clinical Update
6/04/2019
Dangerous Compound Found in CBD Oil
A "CBD oil" was found to contain a dangerous, synthetic cannabinoid. For details, see the Concerns and Cautions section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
May 05, 2022
Seller of CBD Tinctures, Creams & Pet Products Promoted for Pain, Cancer & More Warned by FDA
On February 11, 2022, the FDA issued a warning letter to Rena’s Organic following a review of the company’s social media and website, which found statements about the company’s 300 mg CBD Full Spectrum Oil Cannabinoid Tincture, 600 mg CBD Full Spectrum Oil Cannabinoid ...
Recalls & Warnings
May 09, 2022
FDA Warns Five Companies for Selling CBD Supplements, Gummies and Creams With Delta-8 THC
On May 4th, 2022, the FDA issued warning letters to five companies for selling CBD and other products labeled as containing delta-8 tetrahydrocannabinol (delta-8 THC).
CL Answer
Are there supplements, foods, or beverages that I should avoid when taking clopidogrel (Plavix)?
Learn about supplement interactions with clopidogrel (Plavix). Supplements that may interact with this medication include cannabidiol (CBD), grapefruit, St. John's wort, ginkgo and others.

Clinical Update
4/17/2021
CBD for Low Back Pain?
Does taking CBD help in treating acute low back pain? See what a recent study found in the What It Does section of our CBD & Hemp Extract Supplements, Lotions, and Balms Review. Also see our Top Picks among CBD products.
Clinical Update
6/19/2021
CBD Interaction With Caffeine
Be aware that if you consume caffeine, very high doses of CBD may increase caffeine levels in the body and, potentially, caffeine side effects, according to a new study. Get the details about this and other interactions in the Concerns and Cautions section of our CBD & Hemp Extracts Review. Also see our Top Picks among CBD oils and hemp extracts.
Clinical Update
8/31/2018
CBD & Psychosis
Can giving CBD to people at risk of psychosis help normalize their brain function? Learn what a recent study showed in the What It Does section of the CBD & Hemp Extract Supplements Review. (Also see our Top Picks among recently tested CBD supplements.)
Clinical Update
2/27/2018
Cannabidiol (CBD) for Epilepsy Treatment
Commercially available CBD (cannabidiol) preparations were found to be as effective as a prescription anticonvulsant as an add-on therapy for children with epilepsy in a recent study. CBD was less likely to cause side effects than the drug. Get the details, including dosage, in the What It Does section of the CBD & Hemp Supplements Review (which includes our Top Picks).
Clinical Update
4/10/2018
CBD for Schizophrenia?
People with schizophrenia were given CBD (cannabidiol) twice daily in the hope that it would improve symptoms and cognitive performance. Find out if it helped in the What It Does section of the CBD Supplements Review. (Also see our test results for CBD products.)
Clinical Update
6/26/2018
CBD Approved as Drug
The first drug containing CBD was approved this week by the FDA. The dosage, however, is quite different from that being used by many as a supplement. Get the details in the CBD and Hemp Supplements Review. (Also see our Top Picks among CBD products.)
Clinical Update
3/08/2019
CBD & Cancer
Preliminary evidence suggests that CBD (cannabidiol) may have anti-cancer properties, and a dramatic reduction in tumor size was recently reported in a person taking low-dose CBD. For details, see the What It Does section of the CBD Oils and Hemp Extracts Review.
Clinical Update
Clinical Update
6/22/2019
CBD Interaction
Taking CBD along with anti-seizure medication was recently reported to raise blood levels of the medication by as much as 280%. CBD can interact with many medications. For details, see the Concerns and Cautions section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
7/20/2019
CBD Interactions
CBD can interact with many medications, and we've just added more to the list based on a recently published review of CBD safety. Learn more in the Concerns and Cautions section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
11/17/2018
Rash From CBD Supplement
CBD (cannabidiol) may have caused a serious rash in an individual. For details, see the Cautions and Concerns section of the CBD and Hemp Extract Supplements Review. (Also see our Top Pick for CBD.)
Clinical Update
1/15/2019
Anxiety Treatment -- Low-Dose CBD?
Does low-dose CBD (cannabidiol) help with anxiety and sleep disorders? See the results of a recently published case series in the What It Does section of the CBD & Hemp Extract Supplements Review. Also see our Top Pick for CBD.
Clinical Update
4/19/2019
CBD for Post-traumatic Stress Disorder (PTSD)?
Can moderate doses of CBD help people with post-traumatic stress disorder? See the latest clinical findings in the What It Does section of the CBD Oil and Hemp Extract Supplements Review. Also see our Top Picks for formulations of CBD.
Clinical Update
5/05/2020
CBD for Osteoarthritis Pain
A recent study, conducted in dogs, showed promise for CBD in reducing pain and inflammation due to osteoarthritis (pain due to worn joints). See the details in the What It Does section of the CBD and Hemp Extracts Review. Also see our Top Picks for CBD for people and for pets.
Clinical Update
6/30/2020
CBD for Sleep?
The ability of CBD to improve sleep may depend on the dose given and whether there are underlying conditions causing a sleep problem. See the results of the latest study and learn more in the What It Does section of the CBD and Hemp Extracts Review. Also see our Top Picks for CBD.
Clinical Update
7/02/2019
Best Way to Take CBD
If you use CBD, it's important that you take it in a way that maximizes its absorption. To learn more, see the What to Consider When Using section of the CBD Oils and Hemp Extracts Review, where you'll also see results of a new study showing that how you take CBD may dramatically affect how long it remains in your body. Also see our Top Picks among products.
Clinical Update
10/06/2020
CBD Interaction With Warfarin
High doses of CBD may affect blood clotting and require a dosage adjustment in people taking the blood thinner warfarin, as recently reported. Get the details and learn about other interactions with CBD in the Concerns and Cautions section of our CBD and Hemp Extract Review.
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
CL Answer
I take levothyroxine (Synthroid), a thyroid hormone to treat hypothyroidism. Are there supplements or foods I should avoid, or be taking, due to this drug?
Is taking certain supplements and hypothyroidism medications bad? Learn which supplements can interact with thyroid hormone medications like levothyroxine (Synthroid), thyroid hormone levels, and laboratory tests.
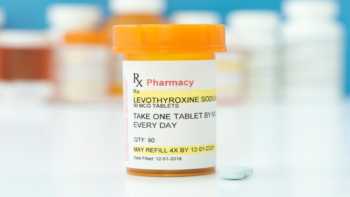
CL Answer
My dog is getting older and his veterinarian recently recommended giving him a glucosamine supplement for his joints. Has ConsumerLab.com tested these, or other supplements for pets?
Find out our test results on glucosamine for dogs and cats as well as other supplements for pets.

Recalls & Warnings
March 09, 2021
CBD Seller Warned for Drug and COVID-19 Claims
On March 1, 2021, the FDA issued a warning letter to Cannafyl following a review of the company's website, which found statements made about the company's products Balance CBD Drops, Relief CBD Drops, Relax CBD Drops and Relief CBD Salve to be drug claims.
Recalls & Warnings
January 10, 2023
Seller of CBD Warned for COVID-19 Claims
On January 5, 2023, the FDA issued a warning to PharmaCanna after review of the company’s website and social media, which found statements about the company’s cannabidiol (CBD) product, CBDefense 2000, to be drug claims because they promoted the products to prevent or treat ...
Recalls & Warnings
January 12, 2023
FDA Warns Two Sellers Promoting CBD to Treat COVID-19
On January 10, 2023, the FDA issued warning letters to two companies following a review that found statements made on the companies’ websites to be drug claims because they promoted the cannabidiol (CBD) products to prevent or treat COVID-19.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
CL Answer
Which supplements may benefit people with essential tremor, and which should be avoided?
Learn about supplements used for treating essential tremor, including caprylic acid (also called octanoic acid), thiamin (vitamin B1), cannabidiol (CBD), GABA (gamma-aminobutyric acid), and branched-chain amino acids. Find out which work and which don't. Also find out which supplements and food may make essential tremors worse.

CL Answer
What are the best supplements for depression and anxiety?
Best natural supplements for depression and anxiety, including the evidence for fish oil, vitamin D, probiotics, and herbal supplements for depression such as curcumin (from turmeric) and saffron.

CL Answer
I've heard that grapefruit juice can interact with medications because it inhibits an enzyme that breaks down drugs in the body. Do any supplements interact the same way with drugs?
CYP3A4 inhibitors, such as grapefruit, can interact with certain medications by inhibiting the liver enzyme that metabolizes many drugs. ConsumerLab.com's answer explains.

CL Answer
How are you staying healthy during the coronavirus pandemic?
Tips for staying healthy during the coronavirus (COVID-19) pandemic. Tips from ConsumerLab members for maintaining physical health, mental health and social well-being during COVID-19.

Recalls & Warnings
September 12, 2024
Root Bioscience Warned for CBD Claims
On August 30, 2024, the FDA issued a warning letter to Root Bioscience Brands, LLC dba Naternal following a review of the company's websites, which found statements made about the company's products, including CBD Oils Move CBD+CBG Oil, Rest CBD+CBN Oil, Nurture Broad Spectrum ...
Recalls & Warnings
November 03, 2022
Seller of CBD Warned for COVID-19 Claims
On November 1, 2022, the FDA issued a warning letter to Alternative Health Distribution LLC (d/b/a CannaAid) following a review of the company’s website, which found statements about the company’s cannabidiol (CBD) products to be drug claims.
Clinical Update
Clinical Update
8/27/2019
CBD Questioned for Epilepsy
Although CBD has been approved in the U.S. for treating seizures associated with rare forms of epilepsy, a review in Europe raised concerns. For details see the What It Does section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
5/30/2020
Is CBD Safe?
Find out the most common adverse events reported for a popular line of CBD products in the Concerns and Cautions section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
10/23/2018
CBD for Anxiety
CBD taken 90 minutes before public speaking reduced anxiety without side effects in a recent clinical trial – although it only worked at one of the three doses studied. For details, see the What It Does section of the CBD and Hemp Extract Supplements Review. (Also see our Top Picks among products.)
Clinical Update
10/08/2019
Does CBD Convert to THC in the Body?
Laboratory studies have raised concerns that CBD could convert to THC (a psychoactive compound in cannabis) after ingestion. A recent study put this to the test in people. See what happened in the What To Consider When Buying and Using section of the CBD Oil and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
8/17/2021
CBD for Burnout?
Healthcare workers were given daily CBD to see if it reduced symptoms of emotional exhaustion and burnout. Did it work and was it safe? See the findings in the What It Does section of our CBD Oil & Hemp Extracts Review.
Clinical Update
3/04/2021
CBD Product Causes Positive Drug Test
A moderately high dose of a full-spectrum CBD product caused positive drug tests in 50% of people in a recent study. See the details in the Concerns and Cautions section of our CBD & Hemp Extract Supplements, Lotions, and Balms Review.
Clinical Update
5/04/2021
CBD for Pain?
Can CBD reduce pain severity or help people better tolerate pain? See what a recent study found in the What It Does section of our CBD & Hemp Extract Supplements, Lotions, and Balms Review.
Clinical Update
9/19/2024
CBD Side Effect
Rare cases of severe, recurrent vomiting and nausea have been associated with daily use of higher-dose CBD, according to recent reports. Get the details in the Concerns and Cautions section of our CBD & Hemp Extract Supplements Review.
Clinical Update
8/12/2022
Thyroid Medication and CBD Interaction
Levothyroxine (a thyroid medication) may interact with CBD. Learn more in our article about Interactions with Levothyroxine (Synthroid).
Clinical Update
6/03/2022
CBD Arthritis Cream?
Did CBD cream decrease pain or improve function in people with arthritis in their hands? See what a recent study found in our CBD Review.
CL Answer
Which supplements can help to lower blood pressure?
Supplements to lower blood pressure, including CoQ10, vitamin C, fish oil, and olive oil, are all explored to find out what works.

Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Product Review
Black Currant, Borage, Evening Primrose, Flax and Hemp Oil Review
Choose the Best Flaxseed and Other Oils Based on Our Tests.

News Release
September 18, 2020
Analysis of CBD Products Shows Price Drop and Lower Levels of THC
White Plains, New York, September 18, 2020 — The recent analysis by ConsumerLab of CBD and hemp extract products revealed a 70% drop in the lowest cost to get CBD since 2018 as well as fewer products containing detectable amounts of THC, a psychoactive compound in hemp.
Recalls & Warnings
July 06, 2021
CBD Products Were Promoted to Treat Cancer and Alzheimer's Without Proof, Says FTC
On July 6, 2021, the Federal Trade Commission (FTC) announced that it has approved a final administrative consent orders against Kushly Industries LLC and the company's owner, Cody Alt, for allegedly making unsupported health claims about its CBD products.
Recalls & Warnings
May 14, 2021
Maker of Care by Design CBD Settles Charges of Unsupported Claims
On May 5, 2021, Cannacraft, Inc.
Recalls & Warnings
July 23, 2019
FDA Warns Seller of CBD Lotions, Patches, Tinctures and Pet Products
On July 22, 2019, the FDA issued a warning letter to Curaleaf, Inc.
CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
CL Answer
Do supplements help with Parkinson’s disease treatment or prevention?
Learn what has been shown with vitamins D and E, niacin, CoQ10, melatonin, creatine, SAMe, NAC, valerian, CoQ10, and CBD, as well as with coffee and the Mediterranean and MIND diets for treating and/or preventing Parkinson's disease.

Recalls & Warnings
August 08, 2022
Seller of CBD Warned for COVID-19 Claims
On August 4, 2022, the FDA sent a warning letter to FluxxLab LLC following a review of the company’s website and social media which found statements about the company’s Covid-19 Immune Support Tincture and CBDA+CBD Oil Tincture products to be drug claims because they ...
Recalls & Warnings
May 16, 2020
CBD Oil Recalled, Risk of High Lead Exposure
On May 15, 2020, Summitt Labs issued a recall of one batch of KORE ORGANIC Watermelon CBD Oil Tincture due to contamination with lead. A random sample of this product tested by the Florida Department of Agriculture and Consumer Services found it to contain lead levels at 4.7 ppm.
Clinical Update
9/18/2021
Help for Arthritic Hands?
A study tested whether moderate doses of CBD could reduce the pain of hand osteoarthritis or psoriatic arthritis. See what the study found in the Pain section of our CBD Oil and Hemp Extracts Review.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
October 22, 2019
Company Marketing CBD for Use in Infants and Children Gets Strong Warning
On October 10, 2019, the FDA and FTC issued a joint warning letter to Rooted Apothecary LLC for "illegally selling unapproved products containing cannabidiol (CBD) online with unsubstantiated claims" including that the products could treat teething pain and ear aches in infants, autism, ...
Recalls & Warnings
June 23, 2020
Seller of CBD Tinctures and "Immune Boost Packs" Warned for Coronavirus Claims
On June 18, 2020, the FDA issued a warning letter to Project 1600 Inc. for selling CBD products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 23, 2020
BIOTA Biosciences Recalls Injectable CBD and Curcumin Products
On May 20, 2020, BIOTA Biosciences LLC issued a recall of five lots of three injectable products because they were marketed without FDA approval.
Clinical Update
11/12/2019
Help for Jaw Pain?
Can CBD ointment relax and reduce pain in the jaw muscles of people who grind their teeth? See what a recent clinical study found in the What It Does section of the CBD and Hemp Extract Review. Also see our Top Picks among products.
Clinical Update
9/07/2019
CBD Missing in Some Products
A class action lawsuit was filed against a popular brand after independent tests showed its products to contain much less CBD than listed. See the Quality Concerns section of the Review for details.
Clinical Update
9/19/2018
Pain and Cannabinoids
Many people use CBD to alleviate pain. This benefit has yet to be proven, but a recent analysis of clinical studies with cannabinoids may explain this effect, as discussed in the What It Does section of the CBD and Hemp Extracts Review. (Also see our Top Picks among these products.)
Clinical Update
5/22/2019
Reducing Drug Craving
High-dose CBD may help reduce drug cravings in people with heroin use disorder according to a recent study. For details, see the What It Does section of the CBD Oils and Hemp Extracts Review. Also see our Top Picks among products.
Clinical Update
11/09/2019
Supplement for Social Anxiety?
High-dose CBD was given to teenagers with social anxiety. Learn what happened in the Anxiety, PTSD, and Sleep section of the CBD and Hemp Extract Review. Also see our Top Pick among products.
Clinical Update
1/26/2024
Help for Stressed Dogs?
Does CBD reduce stress in dogs during car rides? See what a recent study showed in our CBD Review, and see our Top Picks among products for pets.
CL Answer
Do any supplements help for irritable bowel syndrome (IBS)?
IBS supplements may help reduce irritable bowel symptoms such as pain, diarrhea and constipation. Supplements include probiotics, melatonin, and flaxseed.

Recalls & Warnings
July 21, 2022
Young Living Warned for Promoting Essential Oils to Treat Seasonal Allergies, Kidney Stones & More
On June 10, 2022, the FDA issued a warning letter to Young Living Essential Oils Corporate after an inspection of the company’s website and social media found statements about the company’s essential oil and CBD products to be drug claims.
Recalls & Warnings
May 28, 2020
FDA Warns Sellers of CBD, Colloidal Silver, Essential Oils, and More Promoted to Treat Coronavirus
On May 26, 2020, the FDA issued warning letters to four companies for selling products such as CBD, essential oils, colloidal silver, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 26, 2020
Seller of Vitamin and CBD "NoronaPak" Warned for Coronavirus Claims
On May 21, 2020, the FDA issued a warning letter to Apollo Holding LLC for promoting a kit containing vitamins and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 25, 2020
Injectable Curcumin, CBD Are Not Safe, Warns FDA
On April 9, 2020, the FDA issued a warning letter to BIOTA Biosciences LLC following a review of the company's website, which found statements made about some of the company's, products, including Canabidiol (CBD) Complex, Cannabidiol+Curcumin, and Curcumin Complex to be ...
CL Answer
Do any supplements help for flu?
Which supplements may help prevent or reduce symptoms of colds or the flu, including vitamin D, ginseng, NAC, and elderberry.

Clinical Update
11/29/2022
Cancer Pain
Does CBD reduce cancer pain? Find out in the What It Does section of our Review of products.
Recalls & Warnings
February 13, 2016
Cannabis Compound Not Permitted in Supplements, FDA Warns
On February 4, 2016, the FDA issued warning letters to eight companies selling products containing cannabidiol (CBD), a compound derived from cannabis (also known as marijuana). In the U.S., cannabidiol is not permitted to be sold as an ingredient in dietary supplements.
Recalls & Warnings
April 21, 2020
CanaBD Warned for CBD Coronavirus Claims
On April 16, 2020, the FDA issued a warning letter to Nova Botanix LTD DBA CanaBD for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
July 10, 2020
Seller of Thrive "Anti-Viral" Supplement Barred from Making Unproven Coronavirus and Cancer Claims
On July 10, 2020, the Federal Trade Commission (FTC) barred marketer Marc Ching from making unsubstantiated claims that the supplement Thrive can treat, prevent, or reduce the risk of coronavirus (COVID-19). Thrive consists mainly of vitamin C and herbal extracts.
Recalls & Warnings
August 25, 2020
Seller of CBD and NAC Warned for Coronavirus Claims
On August 19, 2020, the FDA issued a warning letter to Living Senior, LLC for selling CBD and NAC products with unsupported claims that they can treat coronavirus (COVID-19).
CL Answer
I have low blood pressure. Are there any supplements I should avoid?
Find out which supplements can cause decreases in blood pressure, including melatonin, arginine, magnesium, and calcium.

CL Answer
I have IBS and don't want to take medication if I can help it. I've heard that peppermint works for IBS, but is there good research to support this?
Information about using peppermint oil for irritable bowel syndrome (IBS), including clinical evidence, dosage, safety and more.

Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
February 16, 2021
Seller of BioCBD+ Products Warned for COVID-19 and Drug Claims
On February 11, 2021, the FDA issued a warning letter to Evolved Ayurvedic Discoveries, Inc.
Recalls & Warnings
April 11, 2020
FDA Warns Sellers of CBD, Colloidal Silver & Natural Remedies Promoted to Treat Coronavirus
Between April 7 and April 9, 2020, the FDA issued warning letters to five companies for selling products such as CBD, colloidal silver, and natural treatments with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 01, 2020
NeuroXPF CBD Warned for Making Coronavirus Claims
On March 31, 2020, the FDA issued a warning letter to NeuroXPF for selling products with unsupported claims that they can treat coronavirus (COVID-19).
CL Answer
I take warfarin (Coumadin), an anticoagulant drug. Are there supplements I should avoid, or be taking, due to this drug?
Find out which supplements should be avoided when taking blood thinners (anticoagulants) like warfarin (Coumadin), including vitamin K, St. John's wort, and curcumin.

CL Answer
Which supplements should be taken with food?
Find out which supplements should be taken with food, including vitamins A, D, E, and K, fish oil, and CoQ10.

Recalls & Warnings
November 07, 2017
Most CBD Oils, Tinctures, Vapors Labeled Inaccurately
Only 31% of CBD (cannabidiol) extracts sold online were found to contain their listed amounts of CBD, according to research published this week in the Journal of the American Medical Association (Bonn-Miller, JAMA 2017).
Recalls & Warnings
November 01, 2017
Hemp Oil and Cannabidiol (CBD) Marketers Warned by FDA Over Claims
On November 1, 2017, the FDA announced it issued warning letters to four companies promoting products containing cannabidiol (CBD), a compound derived from cannabis (also known as marijuana), for the treatment of cancer, as well as other diseases, such as Alzheimer's disease. In the U.
Product Review
Joint Health Supplements for Pets Review (Glucosamine, Chondroitin, MSM & Boswellia)
See Which Pet Supplements for Joint Health Passed Our Tests and Which Didn't!

CL Answer
Are there supplements I should take, or take differently, when intermittent fasting?
Supplements that may help when intermittent fasting. Information about whey protein, CoQ10, magnesium and others, plus coffee, fiber and more.

CL Answer
With herbal supplements, what is the difference between root powder and root extract? Does it matter?
Learn more about the different forms of herbal supplements, including aloe, boswellia, ginkgo, green tea, and milk thistle.
Recalls & Warnings
July 16, 2019
FDA Warns Seller of Magnesium and CBD
On July 9, 2019, the FDA issued a warning letter to Ceba-Tek, Inc.
Recalls & Warnings
May 19, 2020
FDA Warns Sellers of CBD, Kratom, Vitamin C, and More for Coronavirus Claims
Between May 11 and May 14, 2020, the FDA issued warning letters to five companies for selling products such as vitamin C, immune boosters, kratom, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 02, 2020
Seller of Botanical and CBD Oil Patches Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Santiste Labs LLC for promoting its transdermal patches containing botanical oils and/or CBD with unsupported claims that they can treat coronavirus (COVID-19).
CL Answer
Do hair loss supplements, such as Viviscal, Hair La Vie, and Nutrafol, or topical essential oils work?
Vitamins and supplements that may help with hair loss and thinning, including saw palmetto, beta-sitosterol, protein, iron, and vitamin D.

Recalls & Warnings
October 03, 2024
High THC, Toxic Heavy Metals, and Psilocybin Found in Hemp Products
Over 80% of hemp-derived products purchased at various smoke shops, gas stations and specialty CBD vape shops in Delaware were found to contain higher levels of THC (tetrahydrocannabinol, a psychoactive compound) than expected or permitted by law, according to a secret shopper survey conducted by ...
Clinical Update
3/12/2024
Help for Jaw Pain?
Does CBD reduce pain in people with temporomandibular disorder (TMD) when applied topically? Find out in our Review, which includes our Top Picks.
Clinical Update
3/29/2024
What is CBG (cannabigerol)?
What is CBG and how does it compare to CBD? Find out in our Review, which includes our Top Picks among products.
Clinical Update
1/12/2024
L-Theanine for Fatigue?
Did drinking a beverage containing L-theanine, with or without CBD, reduce fatigue? See what a recent study showed in the Stress, sleep and cognition section of our L-Theanine Review, which includes our Top Picks for L-theanine.
Also see: Which supplements help to improve energy and decrease fatigue?
Clinical Update
8/22/2023
Help for Worry and Anxiety?
Does CBD help to reduce symptoms of worry and anxiety? See what a new study found in our Review, which includes our Top Picks among products.
Clinical Update
8/08/2023
Help for Gastroparesis
Very high-dose CBD was found to reduce symptoms such as vomiting and early fullness in people with gastroparesis – slow movement of foods out of the stomach. Details are in our Review, which includes our Top Picks among products.
Clinical Update
5/16/2023
Melatonin Combination?
Does taking melatonin with CBD increase melatonin’s sleep effect or add side effects? See what a new study found.
Also see: What are the best supplements or lifestyle modifications for sleep, and which can make insomnia worse?
Clinical Update
1/28/2022
CBD To Reduce COVID Risk?
Use of a prescription formula was associated with lower risk of COVID-19 infection in a recent analysis, but there are important caveats to this finding. Get the details in the Colds, Flu, and Viral Infections section of our Review.
Clinical Update
4/12/2022
Relief for Knee Replacement Pain?
After knee replacement, can topical CBD reduce pain and pain medication use, or improve sleep? See what a recent study found in the Update in our Review.
Clinical Update
7/01/2022
Don't Trust CBD Labels
Nearly one-quarter of products that claim “not” to contain THC actually contain it, according to a recent study. This can lead to unexpected problems. Learn more in the "Products on the Market" section of our Review.
Clinical Update
7/12/2022
CBD for Epilepsy?
Did a gel of this ingredient reduce seizures in men and women with the most common form of epilepsy? Find out in the Epilepsy and seizure disorders section of our Review.
Clinical Update
11/16/2019
Supplement for Pain?
A recent study gave a modest daily dose of CBD to people with chronic pain who were taking opioid medication to see if it reduced their pain and need for medication. See the results in the What It Does section of the Review. Also see our Top Picks among products.
Clinical Update
2/15/2020
Help for Substance Abuse?
There is preliminary evidence that high-dose CBD may help people with substance abuse disorder. For details, see the What It Does section of the Review. Also see our Top Picks among products.
CL Answer
Hemp Seeds: Health Benefits, Safety, and What To Consider When Picking a Product
Hemp seeds are promoted for heart health, diabetes, boosting testosterone, and other conditions, but do they work? Find out and learn about potential safety concerns.

CL Answer
Do any supplements, foods, or oils help reduce muscle pain, muscle cramps, or nighttime leg cramps?
Find out which supplements, and topical products such as magnesium creams, can help reduce muscle pain, leg cramps, and nighttime leg cramps, and which may worsen muscle cramps. Also, learn about vitamin and mineral deficiencies and common medications that can cause muscle cramps and nocturnal leg cramps.
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
CL Answer
Are there supplements I should avoid when taking apixaban (Eliquis) or similar blood thinner drugs like Xarelto?
Learn about supplement interactions with apixaban (Eliquis), rivaroxaban (Xarelto) and other blood thinner drugs in the direct factor Xa inhibitors class.
Recalls & Warnings
June 20, 2020
FTC Warns 30 More Companies for Coronavirus Claims
On June 18, 2020, the FTC announced that it sent warning letters to 30 companies for selling products such as immune system boosters, colloidal silver, vitamin C, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
News Release
March 04, 2020
CBD Most Often Used for Pain, Then Sleep, According to CL Survey. Half of Users Think It Works.
White Plains, New York, March 4, 2020 — A recent survey of 9,782 people who use dietary supplements found that 14.5% of respondents purchased CBD (or hemp extract, which is a source of CBD) in 2019. The survey asked those people what they used CBD for and whether or not they thought it worked.
News Release
February 29, 2020
Collagen and Magnesium Rise in Popularity, as Fish Oil and Curcumin Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 29, 2020 — A recent survey of 9,782 people who use dietary supplements shows that collagen (+ 4.1 percentage points), magnesium (+ 2.3 pts) and CBD (+ 2.
Recalls & Warnings
December 02, 2020
FDA Warns Sellers of Unapproved COVID-19 Tests, CBD Products
On November 23 and November 30, 2020, the FDA issued warning letters to Industry Lab Diagnostic Partners and Avazo-Healthcare, LLC, respectively, for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).
Recalls & Warnings
June 17, 2024
Do Not Consume Diamond Shruumz Products, Warns CDC and FDA
On June 12, 2024, the Center for Disease Control and Prevention (CDC), Food and Drug Administration (FDA), and America’s Poison Centers warned consumers not to buy or consume Diamond Shruumz chocolate bars, cones, and gummies.
CL Answer
Are there any supplements I should avoid when taking acetaminophen (Tylenol)?
Find out if there are any supplements that should be avoided when taking acetaminophen (Tylenol).
Recalls & Warnings
June 20, 2020
FDA Warns Companies Selling Unapproved COVID-19 Tests
On June 17, the FDA issued warning letters to three companies for marketing unapproved, adulterated or misbranded antibody tests for coronavirus (COVID-19).
Recalls & Warnings
June 05, 2020
Seller of Silver and Vitamin C Lozenges & More Warned for Coronavirus Claims
On June 1, 2020, the FDA issued a warning letter to Dr.
Recalls & Warnings
July 21, 2020
51 CBD Products Recalled Due to Lead Contamination
On June 23, 2020, InHe Manufacturing, LLC and MHR Brands issued a recall of fifty-one CBD products due to contamination and/or potential with lead. Thirty-six of the products are marketed for people and fifteen of the products are marketed for pets.
Recalls & Warnings
March 09, 2022
FDA Warns Seller of Magnesium, CBD, Herbal Extracts & More
On February 9, 2022, the FDA issued a warning letter to Bea Lydecker’s Naturals, Inc.
Recalls & Warnings
June 27, 2024
“Authentic” Rose Oil Often Is Not
Damask rose essential oil (Rosa × damascene) and products claiming to contain this oil, or “authentic rose oil” may often be adulterated or contain less expensive oils instead, according to a recent bulletin from the American Botanical Association (ABC).
Recalls & Warnings
January 03, 2025
Essential Oil Sold on Amazon Recalled
On January 2, 2025, Euqee Wintergreen Essential Oils, sold on Amazon were recalled because the packaging is not child-resistant and may pose a risk of poisoning for young children.
Recalls & Warnings
September 26, 2014
FDA Warns Seller of Supplements and Chocolate Promoted to Treat Ebola
On September 23, 2014, the FDA issued a warning letter to Natural Solutions Foundation, following a review of the company's websites which found statements made about Silver Sol Nano Silver (also called The Silver Solution) and CBD Organic Dark Chocolate Bars (also called High Potency CBD Hemp Oil) ...
Recalls & Warnings
September 08, 2020
Holistic Healthy Pet Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet following a review of the company's website, which found statements made about some of the company's products, including Doctor's Best for Your Pets, Savvy Holistic Health Pet Essences, ...
Recalls & Warnings
April 22, 2024
Primal Kitchen Avocado Oil Recalled Due to Potential for Glass Bottle to Break
On April 19, 2024, Primal Kitchen issued a recall of approximately 2,060 cases of Primal Kitchen Avocado Oil (750 mL) because the glass bottle may be prone to breaking.
CL Answer
Is topical menthol, such as Biofreeze, safe and effective for pain relief?
Menthol is commonly used in topical creams and gels for pain relief. Find out if it works and if it's safe.
Recalls & Warnings
February 01, 2022
Complaint Submitted to FTC for doTerra Essential Oil COVID Claims
On January 28, 2022, the website truthinadvertising.org submitted a complaint to the Federal Trade Commission (FTC) against doTerra International, LLC. following a series of Zoom calls from the company titled "Protocols for the Current Climate.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
July 18, 2022
CVS, Target, Walgreens, and Other Oral Magnesium Citrate Laxatives Recalled Due to Bacterial Contamination
On July 14, 2022, Magnesium Citrate Laxative Oral Solution Lemon Flavor products manufactured by Vi-Jon, LLC and sold under various store brand names were recalled after testing identified the presence of the bacteria Gluconacetobacter liquefaciens.
News Release
February 25, 2020
Top-rated Vitamin and Supplement Brands and Merchants for 2020 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2020 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,782 responses collected in late November and early December 2019.
Recalls & Warnings
March 02, 2021
FDA Warns Seller of Vitamin C, Silver Spray
On March 1, 2021, the FDA issued a warning letter to Ageless Global, LLC following a review of the company's websites for selling Immunoral, Immune Plus, MD Immune Support Spray, and MD CVK-365 Mouth Spray with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 23, 2020
KBMO Diagnostics Warned for Unapproved COVID-19 Test
On June 17, the FDA issued a warning letter to KBMO Diagnostics, LLC for selling the product COVID-19 Fingerstick Test Kit, an unapproved, adulterated and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
June 23, 2020
North Isle Wellness Center Warned for Coronavirus Claims
On June 19, 2020, the FDA issued a warning letter to North Isle Wellness Center for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
News Release
February 24, 2019
CBD and Collagen Rise in Popularity, as Probiotics and Coconut Oil Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 24, 2019 — A recent survey of 10,931 people who use dietary supplements shows that CBD oil (+6.3 percentage points), collagen (+3.6 pts), and bone broth (+2.
Recalls & Warnings
January 28, 2025
Shatavari Powder Recalled Due to Risk of Lead Contamination
On January 27, 2025, New York Wholesale Group recalled Zaarah Herbals shatavari powder because of potential lead contamination.
Recalls & Warnings
March 10, 2025
Probiotics and Prebiotic Gummies Recalled
On January 17, 2025, Health and Happiness (H&H) LLC.
Recalls & Warnings
April 03, 2024
FDA Warns Ambaya Gold for Promoting Products for Depression, Cancer, & Arthritis
On December 5, 2023, the FDA issued a Warning Letter to Ambaya Gold Health Products, LLC following review of the company’s website and social media, which found statements about the company’s Brain Balance, Immune System Boost, Dentist In A Bottle, Essensiac, Fulvic Green, Silver ...
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
Recalls & Warnings
October 09, 2023
Organifi Settles Charges of Unsupported Claims
Organifi, LLC has agreed to pay $150,000 in civil penalties and $50,000 in investigative costs as restitution to settle charges that the company made unsupported claims that its Gold, Pure, Green Juice, and Red Juice products could balance hormone and cortisol levels, regulate the ...
Recalls & Warnings
April 10, 2024
Powerade Variety Packs Recalled
On February 27, 2024, the Coca-Cola Company issued a recall of various lots of Powerade Zero Fruit Punch, Powerade Zero Mixed Berry, and Powerade Mountain Berry Blast electrolyte drinks because the products may contain a foreign metal object, namely a stainless-steel ring.
Recalls & Warnings
March 21, 2024
Eleven Brands of Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On March 19, 2024, Pyramid Wholesale issued a recall of 11 brands of sexual enhancement supplements, including honey-based products, after they were found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
April 24, 2023
FTC Warns Almost 700 Companies: Unsupported Health Claims May Result in Substantial Civil Penalties
On April 13, 2023, the Federal Trade Commission (FTC) notified approximately 670 companies that market over-the-counter (OTC) drugs, homeopathic products, dietary supplements, or functional foods that they could incur significant civil penalties if they make unsubstantiated claims about the ...
Recalls & Warnings
July 26, 2023
Five Leaf Pet Botanicals Warned by FDA for Drug Claims
On June 22, 2023, the FDA issued a warning letter to Five Leaf Pet Botanicals, Inc.
Recalls & Warnings
November 27, 2018
More Dog Food Recalled Due to Risk of Vitamin D Toxicity
On November 27, 2018, Sunshine Mills, Inc. issued a recall of select Evolve Puppy, Sportsman's Pride Large Breed Puppy and Triumph Chicken and Rice Dog Food because they may contain elevated levels of vitamin D.
Recalls & Warnings
November 06, 2018
Dog Food Recalled After Reports of Vitamin D Toxicity
On November 2, 2018, Natural Life Pet Products and Nutrisca issued a recall of certain dry dog foods because they contain elevated levels of vitamin D. The elevated levels of vitamin D were found during an investigation into several reports of vitamin D toxicity by pet owners.
Recalls & Warnings
May 02, 2020
Hopewell Essential Oils Warned for Coronavirus Claims
On April 27, 2020, the FDA issued a warning letter to Hopewell Essential Oils for selling its essential oils with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 10, 2021
Companies Deceived Consumers About Fish Oil for Liver Disease, Says FTC
On April 1, 2021, the Federal Trade Commission (FTC) announced that fish oil supplement manufacturer BASF SE and its U.S.
Recalls & Warnings
July 07, 2020
Sellers of Essential Oils, Hand Sanitizers and More Warned for Coronavirus Claims
The FDA recently issued warning letters to five companies for selling products such as essential oils, homeopathic products, and Chinese herbal products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 08, 2022
Woman Pleads Guilty for Selling and Promoting Products to Treat COVID-19
On July 27, 2022, Diana Daffin, owner of Savvy Holistic Health pleaded guilty to selling products with claims they could treat COVID-19 and promised “Immunity for Humans.” The products were sold under the HAMPL brand name.
Recalls & Warnings
November 21, 2022
6 Supplement Companies Warned by FDA for Making Cholesterol Claims
On May 4, 2022, the FDA issued warning letters to six supplement companies following review that found statements made on company websites and Walmart purchase pages suggesting the products could lower cholesterol to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
November 28, 2022
Seller of Elderberry, Tea Warned for Claims of Treating Cold, Flu, Cancer
On October 18, 2022, the FDA issued a warning letter to Rosebud’s Ranch and Garden, LLC after inspection of the company’s website and social media found statements about the company’s Domestic Divas – Colds and Flu (Tea), Domestic Divas - No Pain No Gain Tea, Green ...
Recalls & Warnings
March 09, 2023
"Dr. Rima Recommends" Nano Silver Recalled
On March 7, 2023, Natural Solutions Foundation issued a recall of Dr. Rima Recommends Nano Silver 10ppm Dietary Supplement, which was promoted to prevent and/or treat COVID-19.
Recalls & Warnings
March 30, 2023
Seller of Tollovid Supplements Warned for COVID-19 Claims
On November 7, 2022, the FDA issued a warning letter to Todos Medical Ltd, aka Todos Medical USA Inc, following a review of the company’s websites, social media, and Amazon storefronts, which found statements about the company’s Tollovid 3CL Protease Inhibitor Delayed Release ...
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
July 14, 2022
FDA Warns Seller of Unauthorized COVID-19 Tests
On June 30, 2022, the FDA issued a warning letter to W.H.P.M, Inc. for distributing COVID antigen tests without approval, clearance, or authorization from the FDA while claiming to mitigate, prevent, treat, diagnose, or cure COVID-19 in people.
Recalls & Warnings
August 03, 2023
Arsenic Poisoning in U.S. from Ayurvedic Supplements
A 75-year-old woman in California experienced arsenic poisoning after taking Ayurvedic supplements prescribed to her by a holistic energy healer, according to a report published in July in Clinical Case Reports.
Recalls & Warnings
May 12, 2020
FDA Warns Seller of "COVID-19 Cough Syrup"
On May 8, 2020, the FDA issued a warning letter to Seanjari Preeti Womb Healing, L.L.C. for selling its product COVID-19 Cough Syrup with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
May 12, 2020
Plum Dragon Herbs Warned for Coronavirus Claims
On May 8, 2020, the FDA issued a warning letter to Plum Dragon Herbs, Inc. for selling Chinese medicine products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
Federal Court Orders Seller to Stop Promoting Silver Product as Coronavirus Cure
On April 29, 2020, a federal court in Utah announced that it has obtained a temporary restraining order preventing Gordon Pedersen and his companies, My Doctor Suggests LLC and GP Silver LLC, from promoting fake treatments for coronavirus (COVID-19).
Recalls & Warnings
August 10, 2020
Seller Indicted for Promoting Silver Product as Coronavirus Cure
On July 28, 2020, Utah resident Gordon Pedersen was indicted by a federal grand jury for posing as a medical doctor to sell an unapproved treatment for coronavirus (COVID-19).
Recalls & Warnings
April 24, 2021
Durisan Recalls Hand Sanitizer Due to Microbial Contamination
On April 10, 2021, Durisan announced a recall expansion of Durisan Antimicrobial Hand Sanitizer, NonAlcohol to include non-expired products because they may be contaminated with the bacterium Burkholderia contaminans.
Recalls & Warnings
July 10, 2018
Blissful Remedies Kratom Recalled Due to Salmonella Risk
On June 30, 2018, Blissful Remedies issued a recall of three kratom products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
June 25, 2020
"Anti-Viral" Supplement Recalled for Unsupported Coronavirus Claims
On June 23, 2020, Golden Nutrition Inc. issued a recall of four lots of Anti-Viral Immune Enhancement Capsules because the label makes unsupported health claims to "help fight corona virus and influenza.
Recalls & Warnings
July 10, 2020
FTC Charges Seller With Falsely Promising Consumers Next Day Shipping of Face Masks and Other PPE
On July 8, 2020, the Federal Trade Commission (FTC) charged the online marketer SuperGoodDeals.com, Inc. and its owner, Kevin J. Lipsitz, with falsely promising consumers next-day shipping of facemasks and other personal protective equipment (PPE) to use during the coronavirus pandemic.
Recalls & Warnings
July 10, 2020
Seller of Hand Sanitizer "Alternative" Warned for Coronavirus Claims
On July 7, 2020, the FDA issued a warning letter to Ionogen, LLC for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 21, 2020
U.S. Attorney's Office Bars Chiropractor from Selling Fake Coronavirus Cures
On April 17, 2020, the United States Attorney's Office for the Northern District of Texas announced that it has obtained a temporary restraining order preventing a chiropractor from promoting fake treatments for coronavirus (COVID-19). Dr. Ray L.
Recalls & Warnings
June 05, 2020
LifeHealth Science Warned for Manufacturing Violations
On May 15, 2020, the FDA issued a warning letter to LifeHealth Science, which found the company's ORËÁ product to be adulterated because it was prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
February 17, 2022
Seller of L-Theanine, Lutein, Noopept and More Warned for Drug Claims
On February 4, 2022, the FDA issued a warning letter to Crystal Clear Supplements following a review of the company’s website and social media which found statements about Tianeptine Sodium Powder, Tianeptine Sulfate Powder, PEA Capsules, Phenibut Capsules, Synephrine Powder, Noopept ...
Recalls & Warnings
August 08, 2022
Recall of Oral Magnesium Laxatives Expanded
On August 3, 2022, Plastikon Healthcare, LLC issued a recall of multiple oral suspension products due to microbial contamination.
Recalls & Warnings
April 17, 2020
Earth Angel Oils Warned for Coronavirus Claims
On April 14, 2020, the FDA issued a warning letter to Earth Angel Oils for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 11, 2020
FDA Warns Seller of Products Promoted to Treat Coronavirus in Pets and Humans
On April 7, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet for selling products intended for pets and humans with unsupported claims that they can treat coronavirus (COVID-19).
News Release
February 15, 2018
Tests Reveal How Much CBD is Really in CBD and Hemp Oils -- Some Don't Provide Amounts of CBD Listed on Labels
White Plains, New York, February 15, 2018 (UPDATED 2/11/19) — CBD (cannabidiol) has become popular for treating pain, helping with anxiety and insomnia, and a variety of other conditions, despite the fact that the FDA has stated it cannot legally be sold as a ...
Recalls & Warnings
February 05, 2019
Hill's Science Diet, Prescription Diet Dog Food Recalled Due to Risk of Vitamin D Toxicity
Recall Expanded (3/20/19): This recall was expanded to include additional products.
Recalls & Warnings
April 14, 2020
Herbs of Kedem Warned for Making Coronavirus Claims
On April 10, 2020, the FDA issued a warning letter to Herbs of Kedem for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Seller of Vitamin C, Vitamin D, Ashwagandha, and More Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Predator Nutrition for selling the products Elixir, Salidroside, Unbreakable, Vitamin C + Bioflavonoids & Rosehip, Vitamin D3, and Ashwagandha with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Seller of Elderberry and Honey Products Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Beepothecary LLC for selling the products BEEHive Delight, BEEbread, and Elderberry, Honey & Propolis Syrup with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Simple Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 23, 2020, the FDA issued a warning letter to Peterson Research Laboratories LLC for selling the product Simple Silver with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
October 19, 2020
Seller of Thrive "Anti-Viral" Supplements Settles with FTC for Unproven Coronavirus and Cancer Claims
On October 19, 2020, the Federal Trade Commission (FTC) approved an administrative consent order to settle charges that marketer Marc Ching was making unsubstantiated claims that the supplement Thrive can treat, prevent, or reduce the risk of coronavirus (COVID-19).
Recalls & Warnings
October 12, 2020
Griffo Botanicals Warned for COVID-19 Claims
On October 7, 2020, the FDA issued a warning letter to Griffo Botanicals for selling various herbal products, including BaseCamp, Gan Mao, Febris, Fortifend, Xiao Chai Hu, HuBei #1, Qing Fei Pai Du, Solis, and WuHan #2, with unsupported claims that they can treat coronavirus ...
Recalls & Warnings
October 12, 2020
Prairie Dawn Herbs Warned for COVID-19 Claims
On October 7, 2020, the FDA issued a warning letter to Prairie Dawn Herbs for selling various herbal products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 02, 2020
Tonic Therapeutic Herb Shop & Elixir Bar Warned for COVID-19 Claims
On September 29, 2020, the FDA issued a warning letter to Tonic Therapeutic Herb Shop & Elixir Bar for selling various herbal products, including Tonic Therapeutic Herb Shop & Elixir Bar, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 29, 2020
FDA Warns Consumers about Fraudulent Tests, Vaccines, and Treatments for COVID-19
On September 21, 2020, the FDA released a statement warning consumers not to buy or use "questionable products" that claim to help diagnose, treat, cure, or prevent coronavirus (COVID-19).
Recalls & Warnings
September 15, 2020
FDA Warns Seller of Unapproved "Wondfo Novel Coronavirus Antibody Detection Kit"
On June 29, 2020, the FDA issued a warning letter to Epro E-Commerce Limited dba DealExtreme and DX.com for selling Wondfo Novel Coronavirus (2019-nCoV) Antibody Detection Kit, an unapproved, adulterated, and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
September 15, 2020
FDA Warns Seller of Unapproved "COVID-19 test package"
On June 29, 2020, the FDA issued a warning letter to Pomegranate Consulting, LLC, Pomegranate Consulting, Ltd. dba Glorious One-Pot Meals for selling COVID-19 test package, an unapproved, adulterated, and misbranded antibody test for coronavirus (COVID-19).
Recalls & Warnings
September 11, 2020
Seller of "COVID PACK" Warned for Coronavirus Claims
On September 9, 2020, the FDA issued a warning letter to Pharmacy Plus, Inc. dba Vital Care Compounder for selling COVID PACK and COVID 'POSITIVE' PACK products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 03, 2020
Seller of "COVID Supplement Protection Pack" Warned for Coronavirus Claims
On June 30, 2020, the FDA issued a warning letter to Center for Wellness and Integrative Medicine for selling the products COVID Supplement Protection Pack (also referred to as the COVID Household Value Pack), Thymosin-Alpha, and Methylene Blue Capsules with unsupported ...
Recalls & Warnings
June 29, 2020
Seller of Chinese Herbal Medicines Warned for Coronavirus Claims
On June 26, 2020, the FDA issued a warning letter to SuperHealthGuard and Loyal Great International Ltd. for selling traditional Chinese medicine products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 14, 2020
Sellers of Miracle Mineral Solution Criminally Charged With Making Coronavirus Claims
On July 8, 2020, federal prosecutors charged the sellers of Miracle Mineral Solution, a toxic bleach solution marketed as a cure for coronavirus (COVID-19), with conspiracy to defraud the United States, conspiracy to violate the Federal Food, Drug and Cosmetic Act, and criminal contempt.
Recalls & Warnings
April 23, 2021
FDA Warns 5 Sellers of Unapproved COVID-19 Tests
Between March 18 and April 6, 2021, the FDA issued warning letters to five companies for selling unapproved, adulterated, and misbranded tests for coronavirus (COVID-19).






